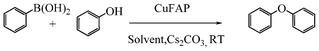
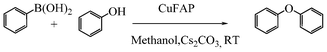
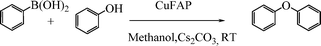
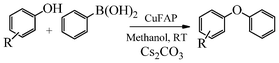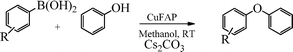Base promoted highly efficient copper fluorapatite catalyzed coupling of phenols with arylboronic acids under mild and ligand-free conditions
Shafeek A. R.
Mulla
*,
Suleman M.
Inamdar
,
Mohsinkhan Y.
Pathan
and
Santosh S.
Chavan
Chemical Engineering & Process Development Division, National Chemical Laboratory, Dr Homi Bhabha Road, Pune-411008, Maharashtra, India. E-mail: sa.mulla@ncl.res.in; Fax: +91-20-25902676; Tel: +91-20-25902316
First published on 19th October 2012
Abstract
A mild, general and highly efficient protocol has been developed for the synthesis of diaryl ethers in good to excellent yield under mild and ligand-free conditions. This is the first example in which a recyclable, heterogeneous copper fluorapatite catalyst is used for the arylation of phenols with arylboronic acids at room temperature in the presence of Cs2CO3 as a base and methanol as a solvent. The catalyst was recovered and reused several times without loss of catalytic activity.
Introduction
Diaryl ethers are key structural motifs (Fig. 1) found in a variety of naturally occurring biologically and medicinally active compounds such as perrottetine (1),1 riccardin B (2),2 and K-13 (3)3. The importance of diaryl ether motifs in cyclic peptide formation has been reviewed by Rama Rao et al.4 The diaryl ether structural motifs play a vital role not only in life science but also in agriculture to prevent various weed-killing chemicals.5 The classic Ullmann coupling reaction for the synthesis of diaryl ethers has been extensively reported, however, its wide application for the synthesis of biologically active molecules containing diaryl ether motifs has been restricted because of the high reaction temperature (125–300 °C) and the long reaction time at which many functional groups are unstable and/or the racemization of the amino acid moieties occurs, resulting in a lower yield of the desired product. Also, the requirement for copper complexes in excess quantities leads to the problem of waste disposal.6 Although significant progress has been achieved,7–14 almost all of the reported methods are not practical approaches for the synthesis of biologically active compounds possessing sensitive functional groups.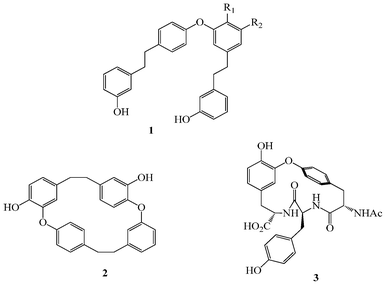 | ||
| Fig. 1 Examples of the diaryl ether motif in natural products. R1 and R2 in 1 are H, OH or OMe. | ||
Arylboronic acid is used to introduce a phenyl ring to various biologically active compounds.15 Also, carbon–heteroatom bond forming reactions of arylboronic acids with amines,16N-hydroxyphthalimides,17 amides, imides,18 and N-heterocycles,19 to give the corresponding products have been reported in the literature. To the best of our knowledge, so far only two research groups, Evans et al.20a and Chan et al.20b have reported the arylation of phenol with arylboronic acid over a Cu(OAc)2 catalyst in the presence of triethyl amine or pyridine as a base and dichloromethane as a solvent. However the coupling of phenol with arylboronic acid described by Evans et al. is advantageous for the synthesis of thyroxine whereas Chan et al. studied N- and O-arylation in which few examples with phenol are reported.
Being that diaryl ether motifs are key constituent of the structural backbone of many pharmaceutical compounds, the construction of their structural units under mild and ligand-free reaction conditions compared to those of the classic Ullmann and Goldberg arylation21 has attracted the attention of researchers world wide. Hence, this paper describes the first report of a recyclable, heterogeneous copper fluorapatite catalyzed coupling reaction of phenols with arylboronic acids in the presence of Cs2CO3 as a base under mild and ligand-free reaction conditions.
As part of our continuous efforts to develop green, ecofriendly, general and cost effective methods for organic transformations,22 herein, we report a highly efficient, cost effective, general and mild method for the synthesis of diaryl ethers in good to excellent yield from the cross coupling reactions of a wide range of substituted phenols with substituted arylboronic acids over an ecofriendly, heterogeneous, reusable, ligand-free copper fluorapatite (CuFAP) catalyst in the presence of Cs2CO3 as a base in a methanol solvent under ambient reaction conditions (Scheme 1) which may be a practical approach for the synthesis of bioactive molecules containing diaryl ether structural units.
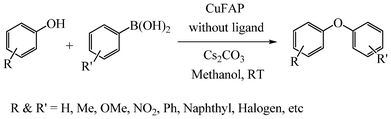 | ||
| Scheme 1 CuFAP catalysed diaryl etherification of substituted phenol with substituted arylboronic acid. | ||
Results and discussion
To develop a protocol for C–O cross coupling reactions, the reaction of phenol and phenyl boronic acid was initially catalyzed by the CuFAP catalyst in the presence of Cs2CO3 as a base in a methanol solvent under ambient conditions to give a 90% yield of biphenyl ether. The results on this substrate under ambient conditions using the ligand-free CuFAP catalyst, prepared as per the literature procedure23 allowed us to select it as a model reaction to optimize the reaction conditions. In order to optimize the reaction conditions, the C–O coupling reaction was performed in various solvents such as methanol, ethanol, dichloromethane, dichloroethane, ethyl acetate, and acetonitrile. Methanol resulted in a high yield of the cross-coupling product, which may be due to it having the most polar and protic character (Table 1, entry 6) as compared to ethanol, dichloromethane, dichloroethane, ethyl acetate, and acetonitrile as solvents, which result in a moderate yield (Table 1, entries 1–5).|
|
||
|---|---|---|
| Entry | Solvent | Yieldb(%) |
| a Reaction conditions: phenyl boronic acid (0.50 mmol), phenol (0.55 mmol), Cs2CO3 (0.75 mmol), solvent (3 ml), catalyst (50 mg), room temperature, 8 h. b Isolated yields. | ||
| 1 | Acetonitrile | 67 |
| 2 | Ethyl acetate | 54 |
| 3 | Dichloromethane | 31 |
| 4 | Dichloroethane | 28 |
| 5 | Ethanol | 79 |
| 6 | Methanol | 90 |
After achieving a high yield in the methanol solvent, studies focused on the investigation of the influence of various bases on the C–O coupling reaction, with Cs2CO3 found to be the most effective base to achieve a high yield (Table 2 entry 7) as compared to NaOH, KOH, Na2CO3, and K2CO3 (Table 2, entries 3–6). The high product yield when using Cs2CO3 as the base may be due to the increasing electropositivity of group IA cations in the order Cs > K > Na, which facilitates the deprotonation of the phenol through the increasing electronegativity of the carbonate salt in the solvent as well as the high solubility of Cs2CO3 in the methanol solvent (Table 2). However, no reaction (N. R.) was observed when using organic bases such as Et3N and pyridine (Table 2, entries 1,2).
|
|
|||
|---|---|---|---|
| Entry | Solvent | Solubility in MeOH (g per 100 mL) at RT | Yieldb (%) |
| a Reaction conditions: phenyl boronic acid (0.50 mmol), phenol (0.55 mmol), base (0.75 mmol), methanol (3 ml), CuFAP (50 mg), room temperature, 8 h. b Isolated yields. c Converted from g per 100 g to g per 100 mL. | |||
| 1 | Triethyl amine | — | N. R. |
| 2 | Pyridine | — | N. R. |
| 3 | NaOH | 23.826a | 24 |
| 4 | KOH | 28.1726b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
31 |
| 5 | Na2CO3 | 0.1726c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
53 |
| 6 | K2CO3 | 4.8426d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
69 |
| 7 | Cs2CO3 | 44.5226c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
90 |
The promising results on the optimized reaction conditions using Cs2CO3 as a base in methanol solvent over the ligand-free CuFAP catalyst encouraged us to investigate the feasibility of this methodology for a wide range of substituted phenols and substituted arylboronic acids for diaryl etherification. As shown in Table 3, substituted phenols possessing a variety of functional groups reacted with phenyl boronic acid with Cs2CO3 as the base in methanol solvent to obtain diaryl ethers as products in moderate to excellent yields. No desired cross-coupling product was obtained under the same reaction conditions in the absence of the CuFAP catalyst (Table 3, entry 1).
|
|
||||
|---|---|---|---|---|
| Entry | Substituted Phenol | Product | Time (h) | Yieldb(%) |
| a Reaction conditions: phenyl boronic acid (0.50 mmol), substituted phenol (0.55 mmol), Cs2CO3 (0.75 mmol), methanol (3 ml), catalyst (50 mg), room temperature, 8 h. b Isolated yields. c No reaction with base (Cs2CO3) without CuFAP catalyst and vice versa. | ||||
| 1 |
|
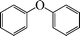
|
24 | N. R.c |
| 2 |
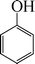
|
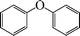
|
8 | 90 |
| 3 |
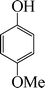
|
|
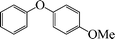
6 | 96 |
| 4 |
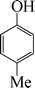
|
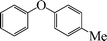
|
6 | 94 |
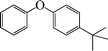
| 5 |
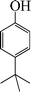
|
|
7 | 92 |
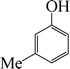
| 6 |
|
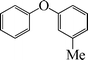
|
8 | 87 |
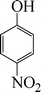
| 7 |
|
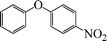
|
24 | 49 |
| 8 |
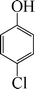
|
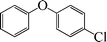
|
12 | 80 |
| 9 |
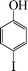
|
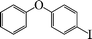
|
12 | 84 |
| 10 |
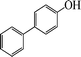
|
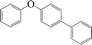
|
10 | 86 |
| 11 |
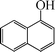
|
|
10 | 84 |
| 12 |
|
|
10 | 82 |
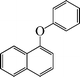
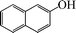
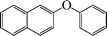
The phenols with electron donating groups such as 4-methoxy phenol, 4-methyl phenol, and 4-tert-butyl phenol, (Table 3, entries 3–5) provided excellent yields as compared to phenol and 3-methylphenol (Table 3, entry 2 and 6) while phenols with electron withdrawing groups such as 4-chlorophenol and 4-iodophenol provided moderate yields after longer reactions times (Table 3, entries 8 and 9) except in the case of 4-nitrophenol (Table 3, entry 7), whereas 4-phenyl phenol, α-naphthol and β-naphthol also provided moderate yields (Table 3, entries 10–12).
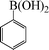
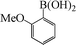
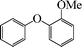
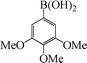
To widen the scope of the methodology using the ligand-free CuFAP catalyst for O-arylation, the coupling of various substituted arylboronic acids with phenol have been investigated; the results are summarized in Table 4. The substituted phenyl boronic acids with electron withdrawing groups such as 4-fluorophenyl boronic acid, 4-chlorophenyl boronic acid, 4-iodophenyl boronic acid and 4-nitrophenyl boronic acid (Table 4, entries 2–5) provided excellent yields as compared to phenyl boronic acid (Table 4, entry 1). However, substituted phenyl boronic acids with electron donating groups such as 3-methylphenyl boronic acid, 4-methylphenyl boronic acid, 2-methoxyphenyl boronic acid, 3,4,5-trimethoxyphenyl boronic acid and 4-methoxyphenyl boronic acid were successfully coupled with phenol to give the corresponding diaryl ethers in moderate yields (Table 4 entries 6–10).
|
|
||||
|---|---|---|---|---|
| Entry | Aryl boronic acids | Product | Time (h) | Yieldb(%) |
| a Reaction conditions: substituted phenyl boronic acid (0.50 mmol), phenol (0.55 mmol), Cs2CO3 (0.75 mmol), methanol (3 ml), CuFAP (50 mg), room temperature. b Isolated yields. | ||||
| 1 |
|
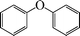
|
8 | 90 |
| 2 |
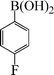
|
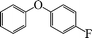
|
6 | 94 |
| 3 |
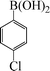
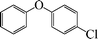
|
|
7 | 92 |
| 4 |
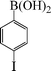
|
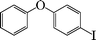
|
8 | 91 |
| 5 |
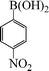
|
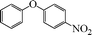
|
8 | 91 |
| 6 |
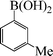
|
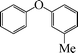
|
9 | 88 |
| 7 |
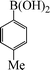
|
|
10 | 84 |
| 8 |
|
|
10 | 82 |
| 9 |
|
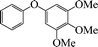
|
12 | 80 |
| 10 |
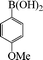
|
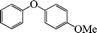
|
10 | 80 |
The results in Tables 3 and 4 indicate that the O-arylation cross coupling reaction is applicable to a large number of substrates having sensitive functional groups; however, the reaction time and the yield obtained are dependent on the nature of the substituents on the phenol as well on the arylboronic acids.
According to previous research work using the CuFAP catalyst for O-arylation,22a N-arylation of heterocycles with chloro- and fluoroarenes23 and N-arylation of heterocycles with bromo- and iodoarenes in the presence of base (K2CO3),24,25 the possible mechanism proposed in Scheme 2 for the C–O cross coupling reaction may involve base promoted CuFAP catalyzed nucleophilic substitution that proceeds via the formation of the complex (A) and then the subsequent oxidative addition of phenyl boronic acid via the formation of another complex (B) followed by instant in situ reductive elimination to release the diaryl ether product (C) as well as the CuFAP catalyst in its original form to be recycled.
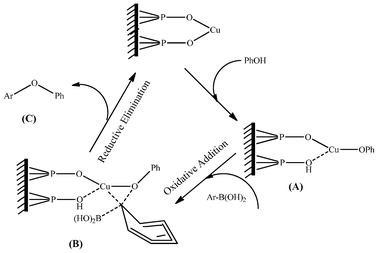 | ||
| Scheme 2 Possible mechanism of the reaction over the ligand-free CuFAP catalyst for diaryl etherification. | ||
Recyclability of copper fluorapatite catalyst (CuFAP)
The recyclability of the ligand-free CuFAP catalyst in the C–O cross coupling reaction was assessed using phenol and phenyl boronic acid with Cs2CO3 as the base in methanol solvent at room temperature; the results are summarized in Table 5. After the completion of the reaction, the catalyst was recovered quantitatively by filtration and recycled several times, however, no loss of catalytic activity was observed even after the fourth cycle (Table 5, entry 5). The catalytic activity of the reused catalyst is very much comparable with that of the fresh catalyst (Table 5, entry 1), which clearly shows that no loss and/or leaching of copper occurred during the course of the reaction and the reused catalyst exhibits an excellent performance.Conclusions
In conclusion, a highly efficient method for the synthesis of diaryl ethers in good to excellent yields from the cross coupling reaction of substituted phenols with substituted aryl boronic acids has been developed over an inexpensive, ligand-free, recyclable, ecofriendly, heterogeneous copper fluorapatite (CuFAP) catalyst in the presence of Cs2CO3 as a base in methanol solvent under ambient reaction conditions. The developed method is mild, general, simple, clean and applicable to a large number of substrates with sensitive functional groups. The CuFAP catalyst was recovered by filtration and recycled several times without loss of catalytic activity.Experimental section
All chemicals and reagents were procured from commercial suppliers and used without further purification. The products were characterized using 1H NMR and 13C NMR spectra. NMR spectra of the products were obtained using a Bruker AC-200 MHz spectrometer with TMS as the internal standard. Column chromatography was performed using silica gel, Merck grade 60–120 mesh size. TLC was performed on 0.25 mm E. Merck precoated silica gel plates (60 F254).General experimental procedure for diaryl etherification
Arylboronic acid (0.50 mmol), phenol (0.55 mmol), Cs2CO3 (0.75 mmol), methanol (3 ml) and CuFAP catalyst (50 mg) were put into a 10 ml round bottomed flask and stirred under a nitrogen atmosphere at room temperature for 6–24 h (Table 3 and 4) and the progress of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was diluted with 10 ml of methanol followed by filtration to recover the catalyst. The filtrate was concentrated in vacuo to get the crude product, which was further purified by column chromatography on silica gel using a hexane/ethyl acetate 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mixture to obtain the diaryl ether product.
10 mixture to obtain the diaryl ether product.
1-Methoxy-4-phenoxybenzene (Table 3, entry 4 and Table 4, entry 10). 1H NMR (200 MHz, CDCl3): δ = 8.54–8.38 (m, 3H), 8.26–8.10 (m, 4H), 7.96 (m, 2H), 3.49 (s, 3H).
13C NMR (200 MHz, CDCl3): δ = 158.35, 139.90, 129.67, 124.71, 123.06, 118.54, 118.22, 115.17, 55.38.
FTIR (CHCl3): 676, 779, 1220, 1492, 1631, 2954 cm−1.
GC-MS: 200, 168, 123, 94, 73, 57.
1-(tert-Butyl)-4-phenoxybenzene (Table 3, entry 6). 1H NMR (200 MHz, CDCl3): δ = 7.25–7.36 (m, 4H), 6.96–7.01 (m, 4H), 6.90 (m, 1H), 2.34 (s, 9H).
13C NMR (200 MHz, CDCl3): δ = 157.56, 154.67, 146.10, 129.63, 126.52, 122.89, 118.43, 34.29, 31.48.
FTIR (CHCl3): 700, 748, 1245, 1501, 1605, 2979 cm−1.
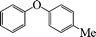
1-Methyl-3-phenoxybenzene (Table 3, entry 7 and Table 4, entry 6). 1H NMR (200 MHz, CDCl3): δ = 7.60–7.49 (m, 4H), 7.36–7.14 (m, 5H), 2.58 (s, 3H).
13C NMR (200 MHz, CDCl3): δ = 157.14, 139.90, 129.67, 124.02, 123.06, 119.54, 118.82, 115.87, 21.38.
FTIR (CHCl3): 698, 738, 1260, 1487, 1607, 3049 cm−1.
GC-MS: 184, 107, 93, 92, 76, 51.
1-Nitro-4-phenoxybenzene (Table 3, entry 8 and Table 4, entry 5). 1H NMR (200 MHz, CDCl3): δ = 8.22 (d, 2H), 7.47–7.25 (m, 3H), 7.10–6.98 (m, 4H).
13C NMR (200 MHz, CDCl3): δ = 163.36, 154.66, 142.59, 130.30, 125.92, 125.40, 120.53, 117.05.
FTIR (CHCl3): 750, 858, 1250, 1370, 1495, 1604, 3058 cm−1.
GC-MS: 215, 138, 122, 76, 51.
1-Chloro-4-phenoxybenzene (Table 3, entry 9 and Table 4, entry 3). 1H NMR (200 MHz, CDCl3): δ = 7.3–7.21 (m, 4H), 7.06 (m, 1H), 6.96–6.86 (m, 4H).
13C NMR (200 MHz, CDCl3): δ = 156.84, 155.92, 129.69, 128.16, 123.61, 120.01, 118.91.
FTIR (CHCl3): 693, 784, 1235, 1496, 1581, 2932 cm−1.
GC-MS: 204, 171, 94, 73, 50.
4-Phenoxy-1,1′-biphenyl (Table 3, entry 11). 1H NMR (200 MHz, CDCl3): δ = 7.03–7.09 (m, 5H), 7.31–7.42 (m, 5H), 7.52–7.58 (m, 4H).
13C NMR (200 MHz, CDCl3): δ = 119, 119.1, 123.35, 126.94, 128.45, 128.80, 129.79, 136.35, 140.64, 156.89.
FTIR (CHCl3): 660, 725, 1240, 1498, 1610, 3021 cm−1.
1-Phenoxynaphthalene (Table 3, entry 12). 1H NMR (200 MHz, CDCl3): δ = 8.16–8.11 (d, 1H, J = 5 Hz), 7.82–7.78 (d, 1H, J = 4 Hz), 7.57 (m, 1H), 7.43 (m, 2H), 7.17 (m, 1H), 7.03–6.86 (m, 6H).
13C NMR (200 MHz, CDCl3): δ = 157.21, 156.57, 152.36, 134.27, 129.14, 127.10, 125.93, 122.48, 121.44, 117.89, 112.86.
FTIR (CHCl3): 670, 790, 1370, 1506, 1619, 3058 cm−1.
Acknowledgements
SMI, MYP and SSC thank CSIR New Delhi for an SRF and JRF, respectively. The authors also thank Dr V. V. Ranade, Chair, CE-PD for encouragement and support.References
- (a) T. Eicher and M. Walter, Synthesis, 1991, 469 CrossRef CAS; (b) T. Eicher, S. Fey, W. Puhl, E. Büchel and A. Speicher, Eur. J. Org. Chem., 1998, 877 CrossRef CAS.
- M. Iyoda, M. Sakaitani, H. Otsuka and M. Oda, Tetrahedron Lett., 1985, 26, 4777 CrossRef CAS.
- J. W. Janetka and D. H. Rich, J. Am. Chem. Soc., 1997, 119, 6488 CrossRef CAS.
- A. V. Rama Rao, M. K. Gurjar, K. L. Reddy and A. S. Rao, Chem. Rev., 1995, 95, 2135 CrossRef.
- The Pesticide Manual, 10th edn; ed. C. Tomlin, Crop Protection Publications: Farnham, UK, 1994 Search PubMed.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1904, 37, 853 CrossRef; (b) J. Lindley, Tetrahedron, 1984, 40, 1433 CrossRef CAS; (c) A. A. Moroz and M. S. Shvartsberg, Russ. Chem. Rev., 1974, 43, 679 CrossRef.
- (a) F. Banaskar, V. Engels, N. Patil, E. V. Rebrov, J. C. Schouten and E. H. Wheatley, Tetrahedron Lett., 2010, 51, 248 CrossRef; (b) S. M. Agawane and J. M. Nagarkar, Tetrahedron Lett., 2011, 52, 5220 CrossRef CAS.
- M. A. Khalilzadeh, A. Hosseini and A. Pilevar, Eur. J. Org. Chem., 2011, 1587 CrossRef CAS.
- Ullmann reaction with use of stoichiometric amount of copper: (a) B. H. Lipshutz, J. B. Unger and B. R. Taft, Org. Lett., 2007, 9, 1089 CrossRef CAS; (b) B. Sofia, M. Florian, W. Michel, B. Catherine, O. Fouad and T. Marc, Green Chem., 2009, 11, 1121 RSC; (c) N. R. Jogdand, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 4019 CrossRef CAS; (d) J. W. Chang, S. Chee, S. Mak, P. Buranaparasertsuk, W. Chavasiri and P. W. Chan, Tetrahedron Lett., 2008, 49, 2018 CrossRef CAS.
- (a) A. V. Kalnin, J. F. Bower, P. Riebel and V. Snieckus, J. Org. Chem., 1999, 94, 2986 CrossRef; (b) R. F. Pellon and M. L. Docampo, Synth. Commun., 2003, 33, 921 CrossRef; (c) Y. T. Luo, J. X. Wu and R. X. Ren, Synlett, 2003, 1734 CAS; (d) A. Cwik, Z. Hell and F. Figueras, Org. Biomol. Chem., 2005, 3, 4307 RSC.
- (a) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (b) Q. Zhang, D. Wang, X. Wang and K. Ding, J. Org. Chem., 2009, 74, 7187 CrossRef CAS; (c) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS.
- (a) M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mazumdar, Tetrahedron Lett., 2007, 48, 8883 CrossRef CAS; (b) T. Miao and L. Wang, Tetrahedron Lett., 2007, 48, 95 CrossRef CAS; (c) J. Zhang, Z. Zhang, Y. Wang, X. Zheng and Z. Wang, Eur. J. Org. Chem., 2008, 5112 CrossRef CAS.
- (a) G. Mann and J. F. Hartwig, Tetrahedron Lett., 1997, 46, 8005 CrossRef; (b) X. Li, X.-Y. Yan, H.-H. Chang, L.-C. Wang, Y. Zhang, W.-W. Chen, Y.-W. Li and W.-L. Wei, Org. Biomol. Chem., 2012, 10, 495 RSC; (c) L-X. Shao and M. Shi, Org. Biomol. Chem., 2005, 3, 1828 RSC; (d) M. Hussain, R. A. Khera, N. T. Hung and P. Langer, Org. Biomol. Chem., 2011, 9, 370 RSC.
- (a) G. Mann, C. Incarvito, A. L. Rheingold and J. F. Hartwig, J. Am. Chem. Soc., 1999, 121, 3224 CrossRef CAS; (b) A. Aranyos, D. W. Old, A. Kiyomori, J. P. Wolfe and S. L. Buchward, J. Am. Chem. Soc., 1999, 121, 4369 CrossRef CAS; (c) S. Harkal, K. Kumar, D. Machalik, A. Zapf, R. Jackstell, F. Rataboul, T. Riermeier, A. Monsees and M. Beller, Tetrahedron Lett., 2005, 46, 3237 CrossRef CAS.
- (a) S. Mori, M. Nagata, Y. Nakahata, K. Yasuta, R. Goto, M. Kimura and M. Taya, J. Am. Chem. Soc., 2010, 132, 4054 CrossRef CAS; (b) J. Wang, C. Medina, M. Radomski and M. J. F. Gilmer, Bioorg. Med. Chem., 2011, 19, 4985 CrossRef CAS; (c) K. Dodo, A. Aoyama, T. Noguchi-Yachide, M. Makishima, H. Miyachia and Y. Hashimoto, Bioorg. Med. Chem., 2008, 16, 4272 CrossRef CAS; (d) A. Speicher, G. Matthias, M. Hennrich and A.-M. Huynh, Eur. J. Org. Chem., 2010, 6760 CrossRef CAS; (e) B. Kilitoglu and H.-D. Arndt, Synlett, 2009, 720 CAS; (f) E. Ullah, J. McNulty, C. Kennedy and A. Robertson, Org. Biomol. Chem., 2011, 9, 4421 RSC.
- J. C. Antilla and S. L. Buchwald, Org. Lett., 2001, 3, 2077 CrossRef CAS.
- H. M. Petrassi, K. B. Sharpless and J. W. Kelly, Org. Lett., 2001, 3, 139 CrossRef CAS.
- D. J. Cundy and S. A. Forsyth, Tetrahedron Lett., 1998, 39, 7979 CrossRef CAS.
- (a) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941 CrossRef CAS; (b) A. P. Combs, S. Saubern, M. Rafalski and P. Y. S. Lam, Tetrahedron Lett., 1999, 40, 1623 CrossRef CAS.
- (a) D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 2937 CrossRef CAS; (b) D. M. T. Chan, K. L. Monaco, R. P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933 CrossRef CAS.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2389 Search PubMed; (b) I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691 CrossRef.
- (a) S. A. R. Mulla, S. M. Inamdar, M. Y. Pathan and S. S. Chavan, Tetrahedron Lett., 2012, 53, 1826 CrossRef CAS; (b) S. A. R. Mulla, A. Sudalai, M. Y. Pathan, S. A. Siddique, S. M. Inamdar, S. S. Chavan and R. S. Reddy, RSC Adv., 2012, 2, 3525 RSC.
- B. M. Choudhary, C. Sridhar, M. L. Kantam, G. T. Venkanna and B. Sreedhar, J. Am. Chem. Soc., 2005, 127, 9948 CrossRef.
- M. L.Kantam, G. T. Venkanna, C.H. Sridhar and K. B. Shiva Kumar, Tetrahedron Lett., 2006, 47, 3897 CrossRef.
- Yao-Bing Huang, Chu-Ting Yang, Jun Yi, Xiao-Jian Deng, Yao Fu and Lei Liu, J. Org. Chem., 2011, 76, 800 CrossRef CAS.
- (a) http://en.wikipedia.org/wiki/Sodium_hydroxide ; (b) http://chemindustry.ru/Potassium_Hydroxide.php ; (c) V. A. Stenger, J. Chem. Eng. Data, 1996, 41, 1111 CrossRef CAS; (d) R. E. Harner, J. B. Sydnor and E. S. Gilreath, J. Chem. Eng. Data, 1963, 8(3), 411 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |