An aqueous anonic/nonionic surfactant two-phase system in the presence of salt. 2. Partitioning of ice structuring proteins
Hua-Neng
Xu
*
State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu 214122, P.R. China. E-mail: hnxujnu@163.com
First published on 5th October 2012
Abstract
Three ice structuring proteins (ISPs) with different molecular weights and net charges at pH 7.4 were partitioned in an aqueous anionic/nonionic surfactant two-phase system of SDS and Trition X-114 in the presence of 100 mM sodium chloride. The effects of temperature and SDS concentration on the protein partition coefficients were investigated. It is found that the protein partition coefficients increase with the increase of temperature and SDS concentration. Compared to the reported net dependence of protein partitioning in aqueous mixed surfactant two-phase systems, the observed protein partitioning behavior is driven primarily by excluded volume interactions between the proteins and the surfactant aggregates, rather than electrostatic interactions. Specifically, the ISPs partition preferentially to the micelle-poor phase, where they experience less excluded-volume interactions with the surfactant aggregates. The improvements in protein partitioning to the micelle-poor phase upon the SDS addition appear to result from the growth of the surfactant aggregates by forming mixed micelles, which enhances the excluded-volume interactions between the proteins and the surfactant aggregates.
Introduction
Ice structuring proteins (ISPs) refer to a class of proteins, which can depress the freezing point of aqueous solutions below the melting point and suppress ice crystal growth. The abilities of ISPs from cold-acclimated plants to inhibit ice growth and reduce the size of ice crystals have an extraordinary significance in the cold storage of food.1–6 The quality loss, such as deterioration in food texture, can be prevented using ISPs by reducing cellular damage and nutrients loss. The classical method adopted for ISP purification from cold-acclimated plants is a combination of conventional processes, such as precipitation, centrifugation, dialysis, ultrafiltration, adsorption, and chromatography, which are generally complicated, time-consuming, and expensive for large-scale production.7–11Aqueous nonionic surfactant solutions exhibit a macroscopic phase separation to form a micelle-rich phase and a micelle-poor phase above a characteristic temperature, called the cloud point.12,13 The phenomenon is attributed to a progressive dehydration of ethylene oxide units in the hydrophilic heads of nonionic surfactants, which has been widely used in the extraction of trace inorganic, organic materials, nanoparticles and biomolecules from different sources.14–21 The cloud point extraction may afford the possibility of large-scale protein purification in a manner that is fast, efficient, and inexpensive compared to traditional protein separation methods such as chromatography, filtration, or centrifugation.15
The tailoring of aggregation and protein partitioning characteristics of nonionic surfactant solutions may be achieved by adding salts, organic solutes, or a second type of surfactant forming the mixed surfactant system.21 The incorporation of ionic surfactants into the nonionic micelles will introduce electrostatic repulsion between the micelles, thus raising the cloud point and hindering the phase separation. However, when electrolytes are added to the mixed surfactant solutions, the original charge distribution is swamped and the corresponding repulsions are screened. This results in a dramatic cloud point lowering.22 In recent years, most of the studies on protein partitioning in aqueous mixed surfactant two-phase systems have been focused on the electrostatic interactions. It was found that the incorporation of ionic surfactant into the nonionic surfactant solutions can introduce electrostatic interactions between the micelles and the proteins and thus attract a desired water-soluble protein into the micelle-rich phase.23
In this study, the partitioning behavior of three ISPs with different molecular weights and net charges at a specific pH, in aqueous mixed surfactant two-phase systems of Triton X-114 (TX-114) and SDS in the presence of NaCl has been investigated. The nonionic surfactant of TX-114 is focused, since it is commercially available and cost-effective. Moreover, it has been used extensively to extract and separate biomolecules.19,20,24,25 This, in turn, will assist in the practical implementation of aqueous two-phase surfactant system as a useful technique for the separation or purification of ISPs. It is clear that mixing surfactants should provide much greater flexibility to alter the properties of the aqueous surfactant two-phase system, in order to attain an improved partitioning of proteins. Moreover, from a practical point of view, using surfactant mixtures is also advantageous economically.
Experimental methods
Materials and solution preparation
Triton X-114 ((1, 1, 3, 3-tetramethylbutyl) phenyl-polyethylene glycol) was purchased from Aladdin, China, and used without further purification. SDS and NaCl was purchased from Sinopharm Chemical Reagent Co. The ISP samples with different molecular weights (MW) and isoelectric point (pI), ISP1 (20.2 kDa, pI 9.1), ISP2 (26.3 kDa, pI 7.2) and ISP3 (66.1 kDa, pI 5.2) from Ligustrum lucidum Ait leaves, were purified using ice-affinity, chromatography separation on a DEAE-cellulose-32 column and a Sephadex G100 column.26All solutions were prepared by weighing the components, and the samples were dissolved in 10 mM Tris-HCl buffer (pH 7.4), which gives the ISPs in the form of one cationic, one neutral and one anionic protein, respectively. The solution was mixed for 24 h using a magnetic stirrer to ensure a perfect homogeneity. The concentration of TX-114 and NaCl was fixed at 100 mM, as it is often used for cloud point extraction. The concentration of SDS was 0, 1 and 2 mM, respectively. The chosen SDS concentration can keep cloud point of the solutions near room temperature.
Protein partitioning measurement
The partitioning experiments were conducted at a very low ISP concentration (0.5 g L−1). At this concentration, the phase separation of the ISP-free surfactant solution is negligibly affected by the presence of the proteins.16 It suggests that the protein-protein interactions, as well as the effects of the proteins on the shape and size of the surfactant aggregates, should be minimal and therefore can be ignored.A definite amount of aqueous surfactant solution containing protein in 10 mM Tris-HCl buffer (pH 7.4) was placed into a calibrated tube. Each partitioning experiment was conducted at a specific temperature at which the micellar system exhibited phase separation behavior. The samples were left to equilibrate in the water baths for 24 h to obtain visually clear phases. The aqueous and micellar phases appeared as top and bottom phases, respectively. After separation, the top and bottom phases of each sample were collected for protein quantification.
The protein concentrations were determined by high performance liquid chromatography (the VP-ODS C18 column, 150 mm × 4.6 mm). The separation was performed using methanol–water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) as a mobile phase. The column temperature was held constant at 35 °C. The flow-rate was 1 mL min−1. The wavelength for the UV detector was set at 280 nm. The protein concentrations were calculated from the integrated peaks using a calibration curve.
30, v/v) as a mobile phase. The column temperature was held constant at 35 °C. The flow-rate was 1 mL min−1. The wavelength for the UV detector was set at 280 nm. The protein concentrations were calculated from the integrated peaks using a calibration curve.
The protein partition coefficient, Kp, as defined in eqn (1), was measured and calculated from the protein concentrations in the top (micelle-poor) and bottom (micelle-rich) phases.
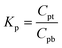
| (1) |
Results and discussion
Fig. 1–3 show the effect of SDS concentration on partition coefficients of the three ISPs (ISP1: MW 20.2 kDa, pI 9.1; ISP2: MW 26.3 kDa, pI 7.2; ISP3: MW 66.1 kDa, pI 5.2) in the aqueous surfactant two-phase systems as a function of temperature. The partition coefficient of the protein is a sensitive parameter that is useful for investigating the balance between repulsive and attractive interactions between proteins and surfactant aggregates in aqueous surfactant two-phase systems.27 Proteins that associate with the surfactant aggregates are expected to show a preference for the micellar phase, whereas proteins with no attractive interaction with the surfactant aggregates or depleted from the aggregates surface generally remain in the less crowded aqueous phase. Here, for all the conditions tested, the partition coefficients obtained are clearly above 1.56, indicating that the ISPs are preferably located in the top aqueous phase. The partitioning of the ISPs into the aqueous phase is enhanced with the increase of temperature and ISP molecular weight. These findings are consistent with the work of partitioning of hydrophilic proteins in aqueous nonionic surfactant two-phase systems by Blankschtein and coworkers.16 It was shown that excluded-volume interactions between hydrophilic proteins and the micelles play the dominant role in experimentally observed partitioning behavior of proteins. Specifically, since hydrophilic proteins tend to remain in the aqueous domain external to the micelles when placed in aqueous surfactant two-phase systems, their partitioning behavior is a function of the difference in free volumes between the two coexisting micellar phases. In that case, larger proteins partition more extremely into the micelle-poor phase where they experience less repulsive excluded-volume interactions with the micelles.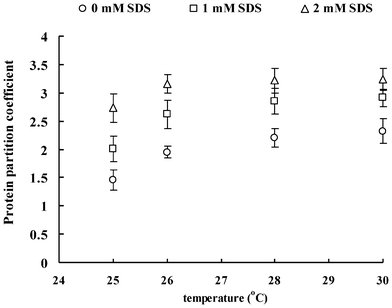 | ||
| Fig. 1 Partition coefficients of ISP1 in aqueous surfactant two-phase systems as a function of temperature. | ||
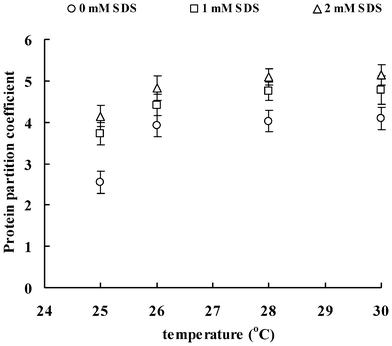 | ||
| Fig. 2 Partition coefficients of ISP2 in aqueous surfactant two-phase systems as a function of temperature. | ||
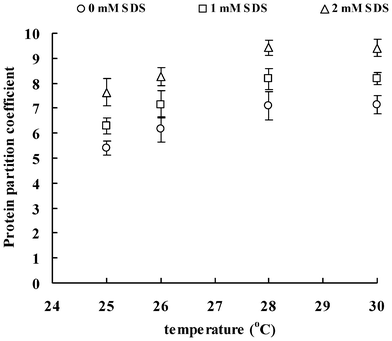 | ||
| Fig. 3 Partition coefficients of ISP3 in aqueous surfactant two-phase systems as a function of temperature. | ||
Of particular relevance to the current investigation is the previously observed influence of SDS addition on the aggregation and phase separation behavior of aqueous TX-114 solutions in the section I. Results of turbidity, DLS and rheo-SALS indicate that the phase separation temperature decreases with the increasing SDS concentration. The hydrodynamic radius, characteristic length and aspect ratio of the surfactant aggregates increase with the SDS concentration, which indicates that the surfactant aggregates can grow in the flow direction and these elongated aggregates can align under shear. The growth of the surfactant aggregates arises from the interactions between the TX-114 and SDS molecules, which then should bring the obvious excluded volume interactions with the ISPs and impel the ISPs partitioning to the micelle-poor phase according to the size of the ISPs. As a result, the partition coefficients of the ISPs due to the enhanced excluded-volume effects become greater with the increasing temperature and SDS concentration.
It is worth noting that, besides the above excluded-volume interactions between ISPs and surfactant aggregates, the addition of SDS to the solutions may bring about electrostatic interactions. In other words, SDS would form mixed surfactant aggregates with TX-114, which provides electrostatic interactions between the surfactant aggregates and proteins. To compare the contribution of the excluded-volume interactions with that of the electrostatic interactions directly, the three ISPs with different pI have been used. At a pH of 7.4, ISP1 and ISP3 are cationic and anionic, respectively, while ISP2 is nearly neutral. Attractive interaction between ISP1 and the anionic mixed surfactant aggregates is considered to result in an increased partitioning to the micellar phase. On the other hand, repulsive interaction between ISP3 and the mixed surfactant aggregates should contribute to the protein partitioning to the aqueous phase. In other words, increasing SDS concentration should give decreasing partition coefficients of ISP1 and increasing partition coefficients of ISP3. However, the net dependence of the protein partition coefficient is not found in the systems, and all the partition coefficients of ISPs increase with the increasing temperature and SDS concentration, as shown in Fig. 1–3. The results from the protein partitioning experiments are in accordance with the findings discussed in the previous sections, that is, in the presence of NaCl, the original charge distribution is swamped and the corresponding electrostatic interactions between the proteins and the surfactant aggregates are screened. As a consequence, the electrostatic interactions between the surfactant aggregates and ISPs are weak, and therefore can be ignored.
Based on the above results, it can be found that the SDS addition is an important factor which affects two aspects of the aqueous anionic/nonionic surfactant two-phase system in the presence of salt. First, it affects the process of surfactant aggregation, and the microstructures of the mixed surfactant aggregate in the solutions. The characteristic length and aspect ratio of the surfactant aggregates increase significantly after the addition of SDS. Second, it is also a critical factor affecting the partitioning of the ISPs that comes in contact with the surfactant aggregates. The partition coefficients of the proteins can be manipulated by virtue of the excluded-volume effects that operate between the surfactant aggregates and the proteins. The growth of the surfactant aggregates upon the SDS addition can bring obvious excluded volume interactions with the ISPs and impel the ISPs partitioning to the micelle-poor phase according to the size of the ISPs. It can serve as a useful guideline to optimize protein separations in aqueous mixed surfactant two-phase systems.
Conclusions
The partitioning of three ISPs in an aqueous anionic/nonionic surfactant two-phase system of SDS and Trition X-114 in the presence of 100 mM sodium chloride is assessed. The protein partition coefficients increase with the increase of temperature and SDS concentration. The excluded-volume interactions between the surfactant aggregates and ISPs play important roles in the protein partitioning behavior, while the electrostatic interactions between them are weak. The growth of the surfactant aggregates upon the SDS addition can bring strong excluded volume interactions with the ISPs, which result in the increasing protein partition coefficients. The results suggest that the formation of mixed micelles enables one to control and optimize the partitioning behavior of proteins by increasing the excluded-volume interactions.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (No. 20906039), the Fundamental Research Funds for the Central Universities (No. JUSRP11124), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- M. Griffith and K. V. Ewart, Biotechnol. Adv., 1995, 13, 375–402 CrossRef CAS.
- R. E. Feeney and Y. Yeh, Trends Food Sci. Technol., 1998, 9, 102–106 CrossRef CAS.
- C. Zhang, H. Zhang and L. Wang, Food Res. Int., 2007, 40, 763–769 CrossRef CAS.
- V. Kontogiorgos, H. D. Goff and S. Kasapis, Food Hydrocolloids, 2008, 22, 1135–1147 CrossRef CAS.
- H. N. Xu, W. Huang, C. Jia, Y. Kim and H. Liu, J. Cereal Sci., 2009, 49, 250–253 CrossRef CAS.
- L. Li, Y. Kim, W. Huang, C. Jia and B. Xu, Cereal Chem., 2010, 87, 497–503 CrossRef CAS.
- V. Kontogiorgos, A. Regand, R. Y. Yada and H. D. Goff, J. Food Biochem., 2007, 31, 139–160 CrossRef CAS.
- W. C. Hon, M. Griffith, P. Chong and D. S. C. Yang, Plant Physiol., 1994, 104, 971–980 CAS.
- M. J. Kuiper, C. Lankin, S. Y. Gauthier, V. K. Walker and P. L. Davies, Biochem. Biophys. Res. Commun., 2003, 300, 645–648 CrossRef CAS.
- D. J. Simpson, M. Smallwood, S. Twigg, C. J. Doucet, J. Ross and D. J. Bowles, Cryobiology, 2005, 51, 230–234 CrossRef CAS.
- C. Zhang, H. Zhang, L. Wang, J. Zhang and H. Yao, J. Agric. Food Chem., 2007, 55, 7654–7658 CrossRef CAS.
- M. Corti, C. Minero and V. Degiorgio, J. Phys. Chem., 1984, 88, 309–317 CrossRef CAS.
- G. Zhao and S. B. Chen, Langmuir, 2006, 22, 9129–9134 CrossRef CAS.
- W. L. Hinze and E. Pramauro, Crit. Rev. Anal. Chem., 1993, 24, 133–177 CrossRef CAS.
- F. H. Quina and W. L. Hinze, Ind. Eng. Chem. Res., 1999, 38, 4150–4168 CrossRef CAS.
- Y. J. Nikas, C. L. Liu, T. Srivastava, N. L. Abbott and D. Blankschtein, Macromolecules, 1992, 25, 4797–4806 CrossRef CAS.
- C. L. Liu, Y. J. Nikas and D. Blankschtein, Biotechnol. Bioeng., 1996, 52, 185–192 CrossRef CAS.
- X. Feng, J. Zhang, S. Cheng, C. Zhang, W. Li and B. Han, Green Chem., 2008, 10, 578–583 RSC.
- J. S. Becker, O. R. T. Thomas and M. Franzreb, Sep. Purif. Technol., 2009, 65, 46–53 CrossRef CAS.
- F. Mashayekhi, A. S. Meyer, S. A. Shiigi, V. Nguyen and D. T. Kamei, Biotechnol. Bioeng., 2009, 102, 1613–1623 CrossRef CAS.
- P. Mukherjee, S. K. Padhan, S. Dash, S. Patel and B. K. Mishra, Adv. Colloid Interface Sci., 2011, 162, 59–79 CrossRef CAS.
- L. Marszall, Langmuir, 1988, 4, 90–93 CrossRef CAS.
- D. T. Kamei, D. I. C. Wang and D. Blankschtein, Langmuir, 2002, 18, 3047–3057 CrossRef CAS.
- L. E. G. de la Vara and B. L. Alfaro, Anal. Biochem., 2009, 387, 280–286 CrossRef.
- M. A. O. da Silva and M. A. Z. Arruda, Talanta, 2009, 77, 985–990 CrossRef.
- Y. Cai, S. Liu, X. Liao, Y. Ding, J. Sun and D. Zhang, Food Bioprod. Process., 2011, 89, 98–102 CrossRef CAS.
- A. H. E. Machado, D. Lundberg, A. J. Ribeiro, F. J. Veiga, M. G. Miguel, B. Lindman and U. Olsson, Langmuir, 2010, 26, 13102–13109 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
