Selective adsorption of nitrate esters with nanostructured carbons
Anna R.
Merritt
*a,
Ramakrishnan
Rajagopalan
b and
Nirupam J.
Trivedi
a
a1 Admin Circle, China Lake, California 93555, USA
b270 Materials Research Laboratory, University Park, Pennsylvania 16802, USA
First published on 27th September 2012
Abstract
The ability of nanoporous carbon adsorbents to display size/shape selective adsorption behavior for energetic nitrate ester molecules was examined. Carbon adsorbents with variable pore size and pore volume were prepared from the pyrolysis of polymeric precursors. Liquid nitrate esters including triethylene glycol dinitrate (TEGDN), N-n-butyl-N-(2-nitroxyethyl)nitramine (BuNENA) and butanetriol trinitrate (BTTN) were then sorbed onto the carbon structure. The adsorbate loading and relative binding strength were observed using thermal techniques such as differential scanning calorimetry and thermogravimetric analysis. This study shows that careful control over the carbon structure can endow adsorbents with selectivity for energetic molecules with different size/shape characteristics; this observation could have important implications for the future development of advanced explosive sensing devices.
1. Introduction
Explosive adsorbent materials are employed in a number of applications ranging from environmental remediation to homeland security. However, significant improvement in these applications could be realized if a material that can selectively discriminate various energetic compounds could be incorporated. For example, nanosensors, which have demonstrated great promise for enhancing sensitivity for trace explosive detection, suffer from low reliability due to their inability to speciate analytes.1 It has been suggested that the integration of selective adsorbents that offer chemical speciation, perhaps in a pre-concentrating device, would additionally engender sensors with high selectivity, resulting in fewer false positives.1,2 Selective adsorbents could also be used for environmental clean-up purposes since enhanced concentrating efficiency of explosives on selective adsorbents could be achieved.3–5Several classes of materials show promise for selective interactions with energetic compounds. Certain types of luminescent polymers, which can reversibly detect explosive compounds via fluorescence quenching, demonstrate good chemical selectivity6 but are limited to applications where the adsorbate has strong electron withdrawing groups (e.g. nitroaromatic compounds).7 Certain self-assembled monolayers (e.g. 4-mercaptobenzoic acid8 and aminopropyl triethoxysilane9) and metallic nanoparticle10 and porphyrin decorated carbon nanotubes11 have also shown selective bonding with explosive molecules but are also limited to interactions with aromatic explosive compounds. In a more general approach, highly selective interactions for explosives have been demonstrated for nano-templated (or molecularly imprinted) polymers.12,13 These materials can differentiate compounds by size and shape exclusion since they possess cavities templated to a specific size and shape for a particular molecule of interest. Hence, explosive molecules with a variety of chemistries can be selectively adsorbed, provided that there are sufficient differences in the explosive molecules' size and/or shape to permit or exclude the molecule from entering the templated cavity.
Similar to nano-templated polymers, the separation of adsorbates on nanoporous carbons (NPCs) can be achieved provided that the adsorbates possess sufficiently dissimilar features in terms of size, shape or chemical affinity. Relatively high separation factors of small gas molecules (e.g. O2, N2, H2, CO2, etc.)14,15 have been extensively demonstrated in the literature. The separation of volatile organic compounds (VOCs), sulfur gases, heavy metals as well as many other industrially important separation processes have also utilized carbon as the adsorptive media.16,17 NPCs are especially attractive adsorbent materials due to their high internal surface area, inert chemical nature, mechanical robustness and easy regenerability via the application of heat, pressure, electrical and/or chemical gradients. The physical attributes of NPC materials, which directly influence adsorptive behavior,4 are also highly tailorable. The pore size/distribution, amorphous/graphitic character and presence of active sites can be tuned by exercising control over synthesis conditions18–20 allowing for the fabrication of effective adsorbents for a wide range of applications.
To date, the selective adsorption of energetic molecules on carbon has received relatively little attention, however, a few studies have shown that activated carbons can preferentially retain small explosive molecules over large ones due to the effect of size exclusion and the relatively large binding energy resulting from the entrapment of small molecules in nanoscopic proximity of both pore walls.4,5 In addition to size exclusion based selectivity, active sites on activated carbons (e.g. heteroatoms) can exhibit selective binding with energetic adsorbates. Although, previous studies have established that activated carbons can perform selective adsorption of energetic molecules, the primary aim of these studies was to produce adsorbents with general affinity towards classes of nitroorganics (e.g. nitrate esters and nitroaromatics) rather than study selective interactions between carbon and specific energetic molecules.4,5
NPCs (or carbon molecular sieves) represent a distinct subset of carbon adsorbents that are characterized by a high level of microporosity. Polyfurfuryl alcohol (PFA) derived NPCs have been shown to possess a narrow size distribution of micropores (pores <2 nm).19 The commensurate dimensions of the NPC pores and the molecular dimensions of adsorbates gives rise to a molecular sieving property, whereby adsorbates can be selectively adsorbed by the NPC on the basis of their size and shape. It is believed that PFA derived NPCs should be able to perform very effective speciation of energetic compounds, provided that the pore size of the carbon is commensurate with the molecular dimensions of the energetic adsorbates and that the energetic adsorbates have sufficiently dissimilar molecular size and shape; this ability to speciate energetic compounds would be of vital importance for the eventual application of these adsorbents in sensing technologies.
As a first step towards realizing selective nanosensors and improved remediation adsorbents, the potential of NPCs to effectively speciate nitrate ester energetic compounds is demonstrated. The aim of this work is to produce general structure–property relationships through the study of the carbon nanostructure/chemistry and its effect on energetic molecule adsorption.
2. Materials and methods
2.1 Preparation and characterization of selective adsorbents
Nanostructured carbons were derived from the pyrolysis of polymeric precursors. The carbon adsorbents designated NPC and a-NPC represent nanoporous carbon and activated nanoporous carbon, respectively, and were derived from a polyfurfuryl alcohol (PFA) precursor. The carbon adsorbent designated a-NPC/P was derived from a PFA/polyethylene glycol diacid (PEGDA) polymer blend. PFA polymers were synthesized from the acid catalyzed polymerization of furfuryl alcohol (FA) monomer. The acid catalyst, 0.1 M p-toluenesulfonic acid monohydrate (98.5%, obtained from Sigma-Aldrich), was dissolved in 5 mL of tetrahydrofuran (THF). The acid–THF mixture was then cooled in an isothermal bath to 10 °C and 185 mmol FA was added with a syringe pump at a rate of 10 ml h−1; the polymerization was carried out over 16 h. The polymeric blend precursor for a-NPC/P was made by mixing PEGDA (MW ∼ 600 g mol−1) and PFA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight). The polymer mixture was allowed to stir at 60 °C for 6 h. The polymer resins were then pyrolyzed in a Lindberg Blue horizontal tube furnace under an argon atmosphere at 900 °C for 1 h. Activated carbons (a-NPC and a-NPC/P) were further post-treated with CO2 activation in a horizontal tube furnace at 900 °C as described in a previous reference.20 All carbons were ground to <45 μm particle size before activation treatment to ensure even and reproducible activation. The mass of carbons before and after activation treatment was monitored; a-NPC had a 50% weight loss during activation while a-NPC/P had a 55% weight loss. Graphite powder, obtained from Fisher Scientific, was used without further modification. Pore size distribution of the adsorbents was characterized by both methyl chloride and nitrogen adsorption. The methyl chloride adsorption apparatus and test procedure has been previously described.21 N2 adsorption was carried out in a Micromeritics Tristar autosorber after treating the samples for analysis at 120 °C for 16 h under vacuum. The skeletal density of the adsorbates was measured with helium pycnometry (Micromeritics, 1340). X-ray photoelectron spectroscopy (XPS, Kratos Analytical, Ultra Instrument) of the carbon adsorbents was used to determine the presence of oxygen functional groups on the carbon adsorbents.
1 by weight). The polymer mixture was allowed to stir at 60 °C for 6 h. The polymer resins were then pyrolyzed in a Lindberg Blue horizontal tube furnace under an argon atmosphere at 900 °C for 1 h. Activated carbons (a-NPC and a-NPC/P) were further post-treated with CO2 activation in a horizontal tube furnace at 900 °C as described in a previous reference.20 All carbons were ground to <45 μm particle size before activation treatment to ensure even and reproducible activation. The mass of carbons before and after activation treatment was monitored; a-NPC had a 50% weight loss during activation while a-NPC/P had a 55% weight loss. Graphite powder, obtained from Fisher Scientific, was used without further modification. Pore size distribution of the adsorbents was characterized by both methyl chloride and nitrogen adsorption. The methyl chloride adsorption apparatus and test procedure has been previously described.21 N2 adsorption was carried out in a Micromeritics Tristar autosorber after treating the samples for analysis at 120 °C for 16 h under vacuum. The skeletal density of the adsorbates was measured with helium pycnometry (Micromeritics, 1340). X-ray photoelectron spectroscopy (XPS, Kratos Analytical, Ultra Instrument) of the carbon adsorbents was used to determine the presence of oxygen functional groups on the carbon adsorbents.
2.2 Explosive adsorption experiments
Adsorbents (carbons) were ground and meshed to <45 μm before adsorption experiments to ensure consistent adsorbent particle sizes and good mass transport by virtue of short intraparticle diffusion lengths. Adsorbents were baked at 120 °C for 16 h prior to adsorption experiments then 300 mg of the adsorbent was weighed into a small vial and 1 g of pure, liquid nitrate ester (adsorbate) was added. The mixture was left at 20 °C for 7 days to allow the adsorbate to diffuse into the adsorbent, after which the adsorbent was filtered from the liquid adsorbate and dried on filter paper. In order to eliminate liquid explosive that was neither bound to the adsorbent surface nor adsorbed in the porosity of the adsorbent, the carbons were rinsed several times with acetone and then dried on filter paper. Differential scanning calorimetry (DSC) (TA Instruments, Q20, N2 purge) analysis was used to assess adsorbate penetration. Samples were prepared in closed aluminum pans and ramped to 300 °C at 5 °C min−1 under flowing N2. The presence of an exotherm in the DSC trace of a nitrate ester soaked carbon indicated the decomposition of adsorbed nitrate ester molecules. Thermal gravimetric analysis (TGA, TA Instruments, Q50, N2 purge) was used to estimate the weight percent loading of sorbed nitrate esters on the carbon adsorbents.3. Results
3.1 Characterization of carbon adsorbents
The physical pore structure and chemical functionalities present on carbon adsorbents have been shown to greatly influence its adsorption characteristics.4 Thus, 4 adsorbents with different pore characteristics and surface chemistries were selected for nitrate ester adsorption studies.Nitrogen and methyl chloride adsorption isotherms were used to characterize the porosity of the carbons. The highly microporous nature of NPC and a-NPC adsorbates necessitated the use of a methyl chloride probe in order to reach equilibrium adsorption conditions in a reasonable timescale.21,22
Graphite was selected as the first candidate adsorbent because of its unique structural and chemical characteristics. The small interlayer spacing of graphite (0.335 nm) makes it effectively non-porous to most adsorbates as reflected by its low apparent surface area of 6 m2 g−1 based on N2 measurements as calculated by the Brunauer–Emmett–Teller (BET) equation. NPC was selected as a second candidate adsorbent. As shown in Table 1, methyl chloride adsorption indicates that NPC is primarily microporous (pore diameter <2 nm). The pore size distribution was found to be narrowly centered around 4–5 Å and the cumulative pore volume was measured to be 0.16 cm3 g−1, in good agreement with PFA derived NPCs produced in previous studies.20 The nitrogen surface area of NPC could not be measured due to its large number of ultramicropores which prevented attainment of equilibrium conditions in a practicable period of time. The third adsorbent, a-NPC, was produced from the CO2 activation of NPC with a 50% by weight carbon burn-off during activation. As expected, an increase in the pore size and pore volume was measured by methyl chloride adsorption upon activation; the mean micropore size of a-NPC increased to 6–8 Å while the cumulative pore volume increased to 0.69 cm3 g−1. The fourth adsorbent (a-NPC/P), an activated carbon derived from a PEGDA/PFA precursor, was chosen for its extremely large pore volume and relatively large pore size. a-NPC/P was derived from a polymer blend composed of PEGDA microphases inside a PFA polymer matrix. Favorable enthalpic interaction between PFA and PEGDA results in the formation of a miscible blend at room temperature. However, during pyrolysis, phase separation starts to occur. Since PEGDA is a non-carbonizing polymer, a carbon with both microporosity (arising from the PFA domains) and mesoporosity (arising from the gasification of the PEGDA micro-domains) is obtained during pyrolysis of the blend. Subsequent activation treatment of this carbon results in very high surface area and enlarged pores due to the excellent diffusion of carbon dioxide promoted by the pre-existing mesoporosity of the PEGDA/PFA derived carbon. The average pore size of the a-NPC/P by Barrett–Joyner–Halenda (BJH) analysis of the nitrogen adsorption data is ∼8 Å.
| Adsorbent | Micropore vol. (cm3 g−1) | Total mesopore and micropore vol. (cm3 g−1) | Mean pore size (Å) | Density by He pycnometry (g cm−3) |
|---|---|---|---|---|
| Graphite (by N2) | — | — | Non-porous | 2.18 |
| NPC (by CH3Cl) | 0.15 | 0.16 | 4 to 5 | 1.82 |
| a-NPC (by CH3Cl) | 0.65 | 0.69 | 6 to 8 | 2.28 |
| a-NPC/P (by N2) | 0.77 | 0.91 | ∼8 | 2.27 |
The skeletal density, as measured by helium pycnometry, was also used to characterize the porosity of the carbon adsorbents. The density of the carbons is shown in the far right column of Table 1. The high density of the a-NPC and a-NPC/P samples relative to NPC has been previously observed and has been attributed to the opening of previously inaccessible porosity upon CO2 activation treatments.23
In addition to the pore structure of the carbon adsorbents, the presence of oxygen functional groups on the carbon surface was determined by XPS analysis since oxygen functionalities can provide specific adsorption sites for adsorbates and hence influence the adsorptive behavior of carbons.3 The oxygen content for each of the carbon adsorbents is shown in Table 2.
| Adsorbent | O/C |
|---|---|
| Graphite | 0.0511 |
| NPC | 0.142 |
| a-NPC | 0.0203 |
| a-NPC/P | 0.0206 |
NPC contains the highest amount of oxygen functional groups while the activated carbons display roughly seven times lower oxygen content. The type of oxygen functionality is difficult to assess by XPS analysis24 due to the asymmetry of the O1s peak,25 however, PFA derived carbons typically display carbonyl and lactonic oxygen moieties.26 Nevertheless, the oxygen content was low for all the carbons.
3.2 Nitrate ester adsorption experiments
The three nitrate esters, triethylene glycol dinitrate (TEGDN), N-n-butyl-N-(-2-nitroxyethyl) nitramine (BuNENA) and butanetriol trinitrate (BTTN) were selected as adsorbates for this study. The selection of these compounds was driven by their dissimilar sizes and shapes which should maximize selective adsorption effects. Fig. 1 shows the energy minimized structures (obtained from Chem3D Pro, MM2 force field) of the 3 nitrate esters and their molecular dimensions in the x–y–z coordinate planes.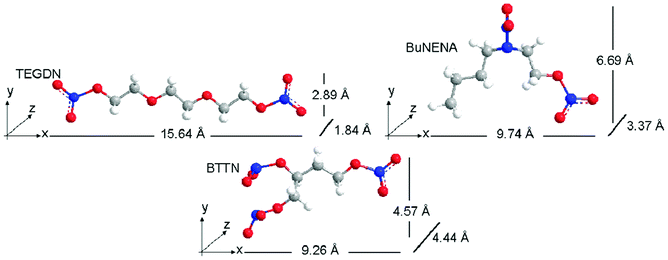 | ||
| Fig. 1 Energy minimized structures of nitrate ester molecules. | ||
TEGDN is characterized by a very long but planar structure while BTTN is very 3-dimensional with significant molecular length along all axes. BuNENA, although fairly large in the x–y plane, possesses a length along the z-axis that is smaller than BTTN but larger than TEGDN. Comparing the dimensions of the z-axis (the smallest dimension) for each nitrate ester molecule, TEGDN is the smallest, followed by BuNENA and BTTN. The ovality of each molecule was also computed using Chem3DPro; an ovality of 1 corresponds to a spherical molecule. The ovalities of BTTN, BuNENA and TEGDN are 1.40, 1.42 and 1.57, respectively.
DSC analysis of the pure nitrate esters adsorbates, shown in Fig. 2, was also performed; all three compounds display a single, strong exothermic peak around 200 °C that corresponds to their thermal decomposition. BuNENA displays an additional smaller exothermic peak around 275 °C.
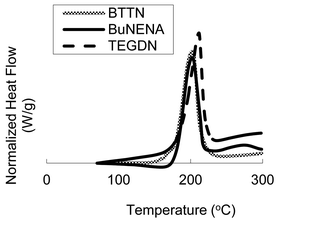 | ||
| Fig. 2 DSC traces of pure nitrate ester adsorbates. | ||
The carbon adsorbents were exposed to the nitrate ester adsorbates to test for selective adsorption. DSC was then used to assess the extent of nitrate ester adsorption for each of the carbon adsorbents. Since the unfilled adsorbents did not display any exothermic peaks up to 300 °C, the presence of a single, strong exothermic peak (see Fig. 3) in the DSC trace of carbons exposed to adsorbates was interpreted as the decomposition of sorbed nitrate esters. Also, since acetone was used to thoroughly rinse the carbons after the adsorption experiments, it is presumed that any nitrate ester detected in the DSC trace is bound to the adsorbent surface (with an energy greater than the solvation energy of acetone) or is adsorbed within the confines of the carbon porosity where solvent displacement would be difficult due to steric considerations.
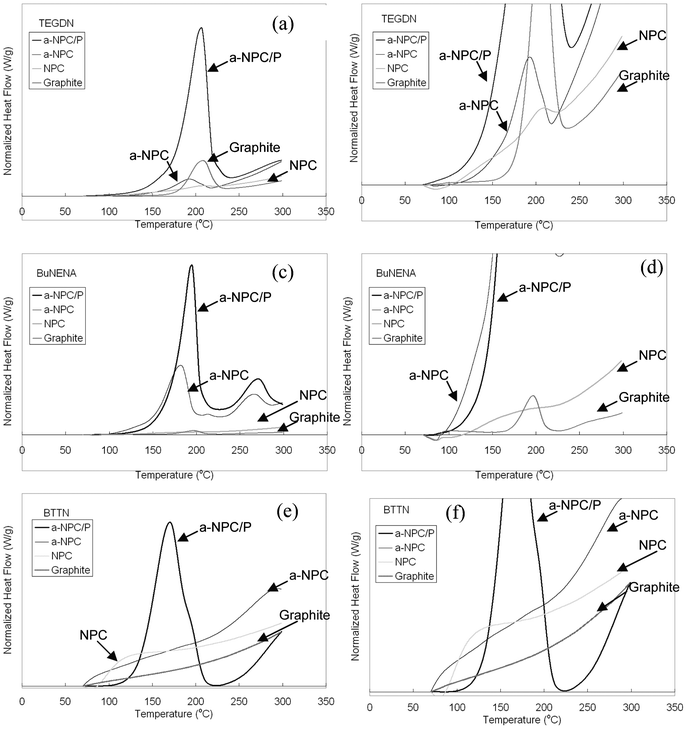 | ||
| Fig. 3 DSC traces of carbon adsorbents exposed to TEGDN (a) and (b), BuNENA (c) and (d), BTTN (e) and (f). | ||
It should be noted that the nanoporous carbon adsorbates under evaluation (NPC, a-NPC and a-NPC/P) have a slit-like pore geometry21 and hence can accommodate molecules into their porosity with dimensions larger than their mean pore size, provided that at least one of the molecular dimensions is smaller than the opening of the slit pore.
The peak decomposition temperatures for the DSC curves shown in Fig. 3 are summarized in Table 3. The pure nitrate ester peak decomposition temperatures are listed in the first line of the table. A dash in Table 3 represents carbon/nitrate ester pairs where there was no measurable adsorption. Interestingly, Table 3 shows that nitrate esters adsorbed onto the carbons have peak decomposition temperatures that are less than the peak decomposition temperatures of the neat nitrate ester, indicating destabilization upon sorption. Since the first and rate determining step in the decomposition of nitrate esters has been established as O–NO2 bond homolysis,27 it is hypothesized that polar interactions between the carbon and the nitrate ester group strain the labile O–NO2 bond and result in lower decomposition temperatures of carbon bound nitrate esters. The amount of shift in the decomposition temperature of the nitrate ester is dependent on the strength of the interaction between adsorbent and adsorbate with stronger interactions causing additional bond strain and hence larger shifts in decomposition temperature.
| Peak decomposition T (°C) | Δ Decomposition temperature; sorbed and pure nitrate ester (°C) | |||||
|---|---|---|---|---|---|---|
| Adsorbent | TEGDN | BuNENA | BTTN | TEGDN | BuNENA | BTTN |
| None | 211.5 | 204.7 | 202.3 | N/A | N/A | N/A |
| Graphite | 207.8 | 196.6 | — | −3.7 | −8.1 | — |
| NPC | 204.8 | — | — | −6.7 | — | — |
| a-NPC | 191.8 | 181.7 | — | −19.7 | −23.0 | — |
| a-NPC/P | 206.5 | 194.7 | 170.1 | −5.0 | −10.0 | −32.2 |
The DSC traces of BuNENA adsorbed onto a-NPC and a-NPC/P both display a second exothermic peak at 270 °C. The second exothermic peak is not believed to be related to the BuNENA decomposition process since the ratios of the main and 2nd exothermic peaks are different for the pure BuNENA and the adsorbed BuNENA DSC traces (see Fig. 2 and Fig. 3). An unidentified impurity in the BuNENA is likely the cause of the second exothermic peak. The impurity is concentrated by the a-NPC and a-NPC/P adsorbates since the peak area of the second exotherm increases relative to the peak area of first exotherm when the compounds are absorbed onto the carbons. The shift in decomposition temperature of the second exothermic peak between the pure and sorbed states is small (∼5 °C) indicating relatively weak interactions with the carbon adsorbate. Given that graphite and NPC do not adsorb any measurable amount of the impurity, presumably due to the absence of porosity in the case of graphite or small pores that excluded the impurity on the basis of its size/shape in the case of NPC, it is believed that the impurity is adsorbed within the porosity of a-NPC and a-NPC/P where solvent displacement is sterically difficult.
The magnitude of the decomposition peaks in Fig. 3 can be used to make a relative comparison of the amount of nitrate ester adsorption for each adsorbent. a-NPC/P displays the largest peak area for all 3 adsorbates. Hence, the large pore volume and pore size of a-NPC/P provides the greatest sorption capacity (of the 4 studied adsorbents) for the nitrate ester adsorbates. TGA experiments were conducted on a-NPC/P adsorbents loaded with BuNENA and BTTN adsorbates to ascertain a rough estimate of the nitrate ester loading. Up to 300 °C there was less than 3% total mass loss for BuNENA and BTTN loaded a-NPC/P, indicating that the overall mass loading of nitrate esters on these carbons is relatively low.
The data in Table 3 were then examined for evidence of selective adsorption.
4. Discussion
The current investigation seeks to describe the effects of adsorbent structure on the adsorption of energetic molecules. Four types of adsorbents were chosen for this study based on the porosity and pore size distribution of the carbons. Graphite was used as a model adsorbent to probe the surface interaction of nitrate esters with carbon. It was found to adsorb TEGDN and BuNENA but not BTTN. The polarizability of the graphite surface has been shown to facilitate adsorption interactions with polar adsorbates.28,29 The interactions of TEGDN and BuNENA with graphite are most likely due to interactions between the polar nitrate ester and nitramine groups and the polarizable basal plane of graphite as shown in Fig. 4a. Adsorption on graphite adsorbents is also known to be highly dependent on adsorbate geometry. Planar adsorbates (or those with a higher ovality), which can approach the graphite surface more closely, may develop stronger interactions with graphite as compared to highly 3-dimensional adsorbates.28,29 This type of selective interaction was evident from the significant binding of TEGDN to the graphite surface as compared to BTTN. Examination of the magnitude of the decomposition peak in Fig. 3 qualitatively illustrates that the graphite, with its low apparent surface area, does not sorb large quantities of nitrate esters relative to the porous carbons.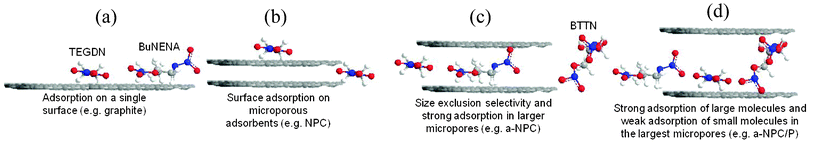 | ||
| Fig. 4 Simplified illustration of how nitrate ester molecules are hypothesized to occupy external or internal adsorption sites on carbon adsorbents. | ||
NPC was found to adsorb only TEGDN. TEGDN adsorbed to NPC displays a negative shift in the peak decomposition temperature and the magnitude of the shift is seen to be comparable to that of nitrate esters sorbed on graphite. A similar magnitude of shift in the peak decomposition temperature of the adsorbed nitrate ester on NPC, coupled with the small relative magnitude of the adsorbed TEGDN decomposition peak (see Fig. 3) suggests that the TEGDN did not have access to the high internal surface area of the NPC microporosity and was excluded based on its large size (relative to the micropores) as shown in Fig. 4b. Given the adsorption of BuNENA on graphite, the lack of measurable BuNENA adsorption on NPC was somewhat surprising but may be related to the very small coherence length of graphite microcrystals in NPC (La in the order of 1.5 nm).23 The absence of long-range ordering of the graphitic microcrystals in NPC results in relatively weak π-interactions between adsorbates and adsorbents30 which, when coupled with the lower ovality of BuNENA, could explain why no measurable sorption of BuNENA on NPC occurred.
a-NPC was found to adsorb TEGDN and BuNENA but not BTTN. The binding energies for TEGDN and BuNENA are significantly larger than for graphite or NPC as interpreted by the very large shift between the peak decomposition temperatures of the a-NPC sorbed nitrate esters and neat nitrate esters (Table 3). a-NPC has a slightly larger pore size with four times the micropore volume of NPC. It is believed that the mode of adsorption of nitrate esters on a-NPC is predominately inside the microporosity rather than on the adsorbent surface as in NPC as shown in Fig. 4c. The strong binding, as inferred from the DSC data, results from the adsorbate experiencing the overlap of adsorption potentials from both pore walls.31,32 Notably, the acceptance of TEGDN and BuNENA into the a-NPC porosity with the exclusion of BTTN, as shown in the middle scenario, is evidence of the ability of nanostructured carbons to discriminate nitrate ester explosives on the basis of their size and shape.
a-NPC/P was found to adsorb all three nitrate esters. Given its large pore volume and pore size, a-NPC/P can admit even the bulkiest (lowest ovality) adsorbate, BTTN. The decomposition of BTTN on a-NPC/P also displays a large negative shift in decomposition temperature due to the strong adsorption potential experienced by the BTTN when it is in close proximity to both pore walls. TEGDN and BuNENA when adsorbed on the a-NPC/P carbon display shifts that are on the same order of magnitude as NPC and graphite, indicating interaction with only a single carbon surface. It is believed that the a-NPC/P pore size is sufficiently large so that smaller adsorbates are relatively far away from the other side of the pore and hence the adsorption potentials do not overlap to cause large shifts in the decomposition temperature. Fig. 4d illustrates how the relatively large micropores in a-NPC/P could result in strong molecular interactions with bulky adsorbates such as BTTN as well as weaker interaction with smaller probes (e.g. TEGDN and BuNENA). Importantly, this observed sensitivity to the molecular fit of the adsorbate inside the adsorbent pore offers the potential to discriminate very small differences in physical dimensions and/or chemical structure even in cases where the adsorbate is not excluded (on the basis of size and shape) from the porosity.
The O/C ratios of the carbons, shown in Table 2, were compared with the DSC results in Table 3 in an attempt to correlate oxygen functionalities to the amount of nitrate ester adsorption and/or the shift in the decomposition temperature of the adsorbed nitrate ester. No correlations between the O/C ratio and sorption behavior were evident. In this study, the effect of large variations in surface areas and pore sizes on the adsorption parameters presumably overwhelms any effect from the very small percentage of oxygen functional groups on the carbon adsorbents.
Conclusions
The adsorption of three different nitrate ester molecules on several types of carbon adsorbents has demonstrated that nanoporous carbons can display selective adsorption properties for energetic molecules. Nitrate esters with large molecular size/shape were excluded from nanoporous carbons with smaller pore sizes (e.g. a-NPC and BTTN) while nitrate esters with smaller size/shape were able to access the carbon porosity (e.g. a-NPC and TEGDN, BuNENA). In all the studied cases, nitrate ester molecules sorbed to the carbon adsorbents displayed a lower peak decomposition temperature than neat (non-sorbed) nitrate ester molecules. This destabilization of the nitrate ester is believed to stem from interactions of the polar nitrate ester group with the polarizable surface of the carbon adsorbents that strain the labile O–NO2 bond, making the nitrate ester molecule more susceptible to thermal decomposition. The magnitude of the shift in decomposition temperature of the sorbed nitrate ester was found to vary depending on the adsorbent properties. In cases where the nitrate ester adsorbate was able to enter the porosity of the carbon adsorbent, relatively large shifts in the peak decomposition temperature of the nitrate ester were observed. The additive effect of the adsorption potentials from both pore walls further straining the labile O–NO2 bond is believed to be responsible for the increased thermal lability of the nitrate ester. Based on these preliminary studies, NPCs are promising materials for explosive sensor technologies; their ability to speciate nitrate ester molecules based on size and shape exclusion as well as by the relative magnitude of binding strength (which is related to the molecular fit of the nitrate ester in the carbon porosity) could potentially be exploited to endow good selectivity to sensor components.It is envisioned that a priori knowledge of the binding strength and size exclusion properties of each adsorbate on each adsorbent would enable the development of very effective electronic nose1 detection schemes.
References
- L. Senesac and T. G. Thundat, Mater. Today, 2008, 11(3), 28 CrossRef CAS.
- R. Xiong, J. T. Fern, D. J. Keffer, M. Fuentes-Cabrera and D. M. Nicholson, Mol. Simul., 2009, 35(10–11), 910 CrossRef CAS.
- R. Leboda, J. Skubiszewska-Zieba, W. Tomaszewski and V. M. Gun'ko, J. Colloid Interface Sci., 2003, 263, 533 CrossRef CAS.
- R. Leboda, V. M. Gun'ko, W. Tomaszewski and B. J. Trznadel, J. Colloid Interface Sci., 2001, 239, 489 CrossRef CAS.
- W. Tomaszewski, V. M. Gun'ko, J. Skubiszewska-Zieba and R. Leboda, J. Colloid Interface Sci., 2003, 266, 388 CrossRef CAS.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- J. C. Sanchez, S. J. Toal, Z. Wang, R. E. Dugan and W. C. Trogler, J. Forensic Sci., 2007, 52(6), 1308 CAS.
- F. Thery-Merland, C. Méthivier, E. Pasquinet, L. Hairault and C. M. Pradier, Sens. Actuators, B, 2006, 114, 223 CrossRef.
- W. M. Heckl, F. M. Marassi, K. M. R. Kallury, D. C. Stone and M. Thompson, Anal. Chem., 1990, 62, 32 CrossRef CAS.
- S. Hrapovic, E. Majid, Y. Liu, K. Male and J. H. T. Luong, Anal. Chem., 2006, 78, 5504 CrossRef CAS.
- X. Lu, Y. Quan, Z. Xue, B. Wu, H. Qi and D. Liu, Colloids Surf., B, 2011, 396 Search PubMed.
- G. M. Murray and B. M. Arnold, Molecularly imprinted polymeric sensor for the detection of explosives, US Patent 6872786, 2005 Search PubMed.
- E. L. Holthoff, D. N. Stratis-Cullum and M. E. Hankus, Sensors, 2011, 11, 2700 CrossRef CAS.
- A. R. Merritt, R. Rajagopalan and H. C. Foley, Carbon, 2007, 45(6), 1267 CrossRef CAS.
- A. F. Ismail and L. I. B. David, J. Membr. Sci., 2001, 193, 1 CrossRef CAS.
- R. C. Bansal and M. Goyal, Activated carbon adsorption, Taylor & Francis, Boca Raton, 2005 Search PubMed.
- M. Smíšek and S. Černỳ, Active Carbon, American Elsevier Publishing Company Inc., New York, 1967 Search PubMed.
- A. Linares-Solano, D. Lozano-Castello, M. A. Lillo-Rodenas, D. Cazorla-Amoros in ed. J.P. Mota and S. Lyubchik, Recent Advances in Adsorption Processes for Environmental Protection and Security, Springer, Netherlands, Dordrecht, 2006, p. 97 Search PubMed.
- C. L. Burket, R. Rajagopalan and H. C. Foley, Carbon, 2007, 45, 2307 CrossRef CAS.
- C. L. Burket, R. Rajagopalan, A. P. Marencic, K. Dronavajjala and H. C. Foley, Carbon, 2006, 44, 2957 CrossRef CAS.
- R. K. Mariwala and H. C. Foley, Ind. Eng. Chem. Res., 1994, 33(10), 2314 CrossRef CAS.
- O. P. Mahajan, Carbon, 1991, 29(6), 735 CrossRef CAS.
- C. L. Burket, R. Rajagopalan and H. C. Foley, Carbon, 2008, 46, 501 CrossRef CAS.
- H. P. Boehm, Carbon, 2002, 40, 145 CrossRef CAS.
- D. Rosenthal, M. Ruta, R. Schlogl and L. Kiwi-Minsker, Carbon, 2010, 48, 1835 CrossRef CAS.
- J. P. Lukaszewicz, Thin Solid Films, 2001, 391, 270 CrossRef CAS.
- M. A. Hiskey, K. R. Brower and J. C. Oxley, J. Phys. Chem., 1991, 95, 3955 CrossRef CAS.
- R. Tachon, V. Pichon, M. Barbe Le Borgne and J-J. Minet, J. Chromatogr., A, 2007, 1154, 174 CrossRef CAS.
- E. Holmgren, H. Carlsson, P. Goede and C. Crescenzi, J. Chromatogr., A, 2005, 1099, 127 CrossRef CAS.
- M. Pérez-Mendoza, M. C. Almazán-Almazán, L. Méndez-Liñán, M. Domingo-García and F. J. López-Garzón, J. Chromatogr., A, 2008, 1214, 121 CrossRef.
- M. C. Almazán-Almazán, M. Pérez-Mendoza, I. Fernandez-Morales, M. Domingo-García and F. J. López-Garzón, J. Chromatogr., A, 2008, 1190, 271 CrossRef.
- E. G. Derouanne, J. M. Andre and A. A. Lucas, J. Catal., 1988, 110, 58 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
