A simple and efficient method for the facile access of highly functionalized pyridines and their fluorescence property studies†
Md. Nasim
Khan
a,
Suman
Pal
a,
Tasneem
Parvin
b and
Lokman H.
Choudhury
*a
aDepartment of Chemistry, Indian Institute of Technology Patna, Patna-800 013, Bihar, India. E-mail: lokman@iitp.ac.in; Fax: +91 612 2277383; Tel: +91 916 122552038
bDepartment of Chemistry, National Institute of Technology Patna, Patna-800 005, Bihar, India
First published on 31st October 2012
Abstract
In this paper, we have described a simple and convenient method for the one-pot multicomponent reaction of aldehydes, malononitrile and thiols in the presence of a catalytic amount of Bronsted base potassium hydroxide for the efficient synthesis of highly functionalized pyridines. The notable features of this protocol are the simple experimental procedure, short reaction time, broad substrate scope and good yields using a catalytic amount of readily available and cheap base. The photophysical behaviours of the synthesized pyridines have been investigated by UV-Vis and fluorescence spectroscopy and some of the synthesized substituted pyridines exhibit promising fluorescence quantum yields.
Introduction
Multicomponent reactions (MCRs) have gained significant attention in recent times due to advantages such as atom, step and pot economy.1 MCRs are considered a vital tool for both combinatorial chemistry and diversity-oriented synthesis.2 Synthesis of functionalized heterocyles by MCRs has become an attractive approach in modern organic synthesis.3 Among the heterocyclic systems, the pyridine nucleus is of considerable significance owing to its presence in biologically active compounds, active pharmaceuticals, broad range of natural products and functional materials.4 For example, diploclidine5 is a recently isolated natural product containing the pyridine core (Fig. 1). Similarly, streptonigrin and lavendamycin are bio-active natural products having a highly substituted pyridine moiety.6 In addition to these, substituted pyridines are prominent building blocks in supramolecular chemistry due to their π-stacking and directed H-bond forming ability.7 | ||
| Fig. 1 Natural products with a substituted pyridine moiety. | ||
Considering the wide applications of functionalized pyridines, the design and development of a new methodology for the synthesis of pyridines have attracted huge attention from both synthetic and medicinal chemists.8 Recently, a plethora of methods for the synthesis of substituted pyridines have been reported in the literature. Among them, regiospecific metallacycle-mediated synthesis,9 nickel-catalyzed dehydrogenative [4 + 2] cycloaddition of 1,3-dienes with nitriles,10 tandem Blaise reaction intermediate and 1,3-enynes,11 coupling of oxime acetates with aldehydes12etc. are notable. Very recently Deiters et al.,13 have synthesized the pyridine core of cyclothiazomycin in a regioselective manner via [2 + 2 + 2] cyclotrimerization as a key step. Substituted 2-amino pyridines tethered with nitrile and amino functionalities as shown in Fig. 2 are considered as privileged medicinal scaffolds.14
 | ||
| Fig. 2 Highly substituted pyridines having potent biological activity. | ||
Literature studies revealed that these penta-substituted pyridines exhibit significant and diverse medicinal properties, such as antibacterial,15 anticancer,16 potassium channel opener for treatment of urinary incontinence,17 anti hepatitis B virus (HBV) infection,18 Parkinson's disease, hypoxia, asthma, cancer and kidney disease19etc. Evdokimov et al.20 reported a multi component reaction of aldehydes, two equivalents of malononitrile and thiophenols for the one-pot synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines using Et3N or DABCO as a catalyst. However, this method suffers from some limitations such as low yields (20 to 48%) and occurrence of by products “enaminonitriles”. The broad pharmacological applications of this moiety inspired the development of a few improved methods using various catalysts such as basic ionic liquid [bmIm]OH,21 piperidine/microwave,22 ZnCl2,23 silica nanoparticle,24 KF/alumina,25 DBU,26 boric acid,27 ZrOCl2·8H2O/NaNH2 in [bmim]BF4 under ultrasound irradiation,28 nanocrystalline magnesium oxide29 and very recently by Zn(II) and Cd(II) metal-organic frameworks (MOFs) etc.30 Most of the literature methods suffer from one or another draw back such as low yields, toxic or expensive catalyst or a longer reaction time. Therefore, development of an improved and generalized method for the synthesis of these privileged molecules using a readily available and inexpensive catalyst has remained important. In continuation of our work on the development of new synthetic methodologies for the synthesis of diverse heterocycles,31 we have observed that the one-pot multicomponent reaction of aldehyde with malononitrile and thiophenol in the presence of a catalytic amount of KOH provides 2-amino-3,5-dicarbonitrile-6-thio-pyridines in good yields (Scheme 1).
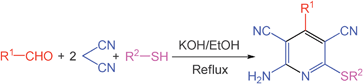 | ||
| Scheme 1 Synthesis of highly substituted 4-aryl pyridines. | ||
It is significant to mention that Xin et al.32 had reported a multicomponent reaction for the synthesis of substituted pyridines tethered with 2-alkoxy group using stoichiometric amount of NaOH (Scheme 2). Whereas herein, we report a four component reaction catalyzed by catalytic amount of either NaOH or KOH for the efficient synthesis of 2-amino, 4-aryl/alkyl pyridine dicarbonitriles by replacing 1,3-dicarbonyl by another equivalent of malononitrile and adding one equivalent of thiol.
 | ||
| Scheme 2 Substrate directed base catalyzed multicomponent reactions for the diversity oriented synthesis of highly functionalized pyridines. | ||
It is noteworthy to mention that previous studies by other groups25,33 reported that this multicomponent reaction was unsuccessful in the presence of sodium hydroxide. It's our surprise that a catalytic amount of NaOH or KOH is useful for the efficient synthesis of functionalized 2-amino pyridine derivatives.
Results and discussion
Initially, the reaction of 4-methoxybenzaldehyde (1.0 equiv.), malononitrile (2.0 equiv.) and thiophenol (1.0 equiv.) was chosen as a model reaction in the presence of 10 mol% base catalyst in ethanol at reflux temperature. Among the readily available bases, we screened this reaction with Na2CO3, KOH, NaOH, NaHCO3 and LiOH (Table 1, entries 2–7). KOH was found to be the most efficient catalyst of choice for this transformation in terms of reaction time and yields obtained. Trace amounts of product were obtained in the absence of catalyst after 20 h reaction time (Table 1, entry 1). Similarly, various solvents like ethanol, acetonitrile, THF, dichloromethane and water were also screened for the same reaction keeping the substrate ratio intact (Table 1, entries 7–11). Among all these solvents, EtOH was found to be the best solvent for this transformation.|
|
||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) |
| a Reaction conditions: 4-methoxybenzaldehyde (3.0 mmol), malononitrile (6.0 mmol), thiophenol (3.0 mmol), catalyst (10 mol%) in ethanol (10 ml) at reflux temperature. b Isolated yields. c Reaction performed at room temperature. | ||||
| 1 | — | EtOH | 20 | Trace |
| 2 | Na2CO3 | EtOH | 1.0 | 62 |
| 3 | NaHCO3 | EtOH | 1.0 | 55 |
| 4 | NaOH | EtOH | 1.0 | 74 |
| 5 | LiOH | EtOH | 1.0 | 62 |
| 6 | KOH | EtOH | 4.5 | 35c |
| 7 | KOH | EtOH | 0.5 | 86 |
| 8 | KOH | CH3CN | 1.5 | 79 |
| 9 | KOH | THF | 1.5 | 35 |
| 10 | KOH | CH2Cl2 | 12 | 30 |
| 11 | KOH | H2O | 2.5 | 35 |
To find the optimum reaction condition, the same model reaction was carried out in the presence of different amounts of KOH (Table 2). The variation of the quantity of KOH from 5 mol% to 30 mol% gave different yields of 1a in 57, 86, 81 and 79% respectively. Higher loading of the catalyst lowered the reaction time but did not improve the reaction yield. Among them the best amount of KOH was 10 mol%.
| Entry | KOH (mol%) | Time (min) | Yield b (%) | |
|---|---|---|---|---|
| a Reaction conditions: 4-methoxybenzaldehyde (3.0 mmol), malononitrile (6.0 mmol), thiophenol (3.0 mmol), catalyst (10 mol%) in ethanol (10 ml) at reflux temperature. b Isolated yield. | ||||
| 1 | 5 | 90 | 57 | |
| 2 | 10 | 30 | 86 | |
| 3 | 20 | 25 | 81 | |
| 4 | 30 | 20 | 79 | |
With the optimized conditions in hand, we turned our attention to investigate the scope and general applicability of this methodology by carrying out the synthesis of pyridines using different aldehydes and thiols (Table 3). We have found that a series of substituted aromatic aldehydes tethered with either electron-withdrawing or electron-donating groups produced 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines in good to excellent yields (Table 3, entries 1, 3–10, 12 and 14–16). Aliphatic aldehydes such as cyclohexanecarbaldehyde also underwent this multicomponent reaction smoothly to provide the corresponding pyridine derivative (1k) in good yield. Similarly, a bulky aldehyde such as 2-naphthaldehyde was also found to be suitable to obtain the corresponding pyridine derivative (1m). Next, the variability of thiols was tested under the given reaction conditions. Benzyl thiol and cyclohexyl thiol were also found to be suitable for this multicomponent reaction to provide corresponding pyridine derivatives (1h, 1i, 1m and 1l respectively) in good to excellent yields. Interestingly, in case of 2-amino thiophenol, the corresponding expected pyridine derivative (1n) was found in very good yield and we did not observe any 2,6-diamino pyridine derivatives. Similarly, 2-methyl thiophenol also provided pyridine derivatives (1o and 1p) in good yields.
|
|
|||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | Producta | Time min/hour | Yieldb (%) |
| a All products were fully characterized by IR, 1H NMR, 13C NMR and elemental analysis. b Isolated yield. | |||||
| 1 | 4-OMeC6H4 | C6H5 | 1a | 30 min | 86 |
| 2 | C6H5 | C6H5 | 1b | 1.0 h | 80 |
| 3 | 4-Cl-C6H4 | C6H5 | 1c | 1.0 h | 81 |
| 4 | 4-Me-C6H4 | C6H5 | 1d | 1.0 h | 85 |
| 5 | 3-Cl-C6H4 | C6H5 | 1e | 45 min | 73 |
| 6 | 4-Br-C6H4 | C6H5 | 1f | 1.0 h | 71 |
| 7 | 4-SMe-C6H4 | C6H5 | 1g | 50 min | 83 |
| 8 | 4-Cl-C6H4 | C6H5CH2 | 1h | 30 min | 83 |
| 9 | 4-Me-C6H4 | C6H5CH2 | 1i | 30 min | 89 |
| 10 | 3-NO2-C6H4 | C6H5 | 1j | 1.0 h | 75 |
| 11 | C6H11 | C6H5 | 1k | 1.5 h | 76 |
| 12 | 4-Cl-C6H4 | C6H11 | 1l | 30 min | 82 |
| 13 | β-C10H7 | C6H5CH2 | 1m | 30 min | 85 |
| 14 | 4-Br-C6H4 | 2-NH2-C6H4 | 1n | 30 min | 90 |
| 15 | 4-Br-C6H4 | 2-Me-C6H4 | 1o | 1.0 h | 90 |
| 16 | 4-CN-C6H4 | 2-Me-C6H4 | 1p | 30 min | 88 |
Interestingly, in case of sterically hindered aldehydes such as 2,6-dichlorobenzaldehyde, 2-chloro-6-fluorobenzaldehyde and 2,6-dimethoxybenzaldehyde, the corresponding expected pyridines were not observed using this methodology. Similar to other literature reported methods,14,20 we also observed the corresponding dihydropyridines (Table 4, entries 1–3). Although, Kantam et al.29 observed the corresponding pyridine derivative in the case of 2,6-dimethoxybenzaldehyde, however in this method, even after a prolonged reaction time we observed only the corresponding dihydropyridine 2c.
All the synthesized molecules were fully characterized using the usual spectroscopic techniques. Among them, for compound 1e, single crystal X-ray crystallographic analysis was done, which further supported our spectroscopic data (Fig. 3).
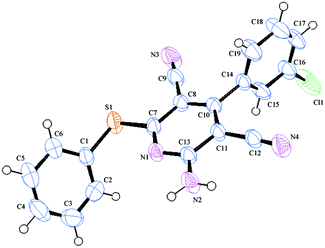 | ||
| Fig. 3 ORTEP plot of compound 1e (CCDC 875077). | ||
After having these successful results, we were interested to see whether this protocol could be extended to other active methylene compounds bearing only one –CN, such as ethyl cyanoacetate as shown in Scheme 3. Unfortunately, the reaction did not afford the desired substituted 2-amino pyridine (1q). Instead, we found the corresponding Knovenegel product (3a) and disulphide (4), which may be due to the lower reactivity of 3a and lower nucleophilicity of the ethyl cyanoacetate.
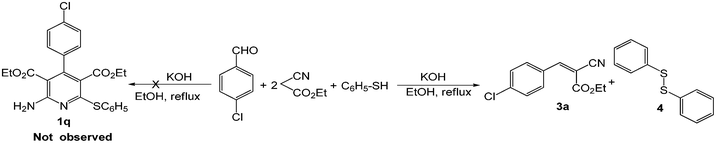 | ||
| Scheme 3 Reaction with ethyl cyanoacetate instead of malononitrile. | ||
Taking cue from the work of Alvarez-Insua et al.,34 we also investigated the possibility of replacing thiols by other nucleophiles such as alcohols and amines using the same catalyst. Interestingly, we found that in the absence of thiol, i.e., the reaction of 4-methoxybenzaldehyde, malononitrile in ethanol and a catalytic amount of KOH, the corresponding expected product 5a was not formed. However, by changing the reaction procedure and increasing the amount of KOH (1 mol. equiv.) we were able to synthesize the corresponding oxo-pyridine derivatives (Table 5, entries 1 and 2). In this reaction, the sequence of the addition of reagents is very crucial, e.g. when all the reactants were mixed at once in the presence of KOH (1.0 equiv.) it provided the corresponding Knoevenagel alkene (3b) along with an unwanted side product (6) (Scheme 4).
 | ||
| Scheme 4 Dependency on the addition sequence of starting materials. | ||
Similar to ethanol and methanol, aliphatic cyclic amines such as pyrrolidine and piperazine provided the corresponding substituted pyridines (Table 5, entries 3 and 4) in good yields. In the case of aromatic amines the reaction was not successful.
A mechanism portraying the probable sequence of events for the synthesis of thiopyridine derivatives is shown in Scheme 5. Condensation of an aldehyde with malononitrile leads to the corresponding Knoevenagel product. Next, the second molecule of malononitrile undergoes Michael addition followed by simultaneous thiolate addition to C≡N of the adduct and cyclization leads to dihydropyridine, which undergoes oxidative aromatization to provide highly substituted pyridine.
 | ||
| Scheme 5 Proposed mechanism for the KOH mediated synthesis of thio-pyridine derivatives. | ||
Photophysical properties
Synthesis of organic compounds with high quantum yield has been the subject of intense study because of their demand as dyes,35 biological markers,36 functional organic devices and sensors,37 as well as in organic light emitting devices (OLEDs). An organic molecular system of a donor-π-electron bridge acts as a molecular rectifier.38 Considering the biaryl structure and extended conjugation with the CN moiety, we were interested to see the optical behaviour of the synthesized highly substituted pyridines containing D-π-A (donor-π system-acceptor) push pull system. Initially, UV-Vis and fluorescence behaviour of compound 1a was investigated at room temperature in different polar protic and aprotic solvents such as chloroform, methanol, acetonitrile and DMSO (Table 6, Fig. 4). Because of solubility problems we could not study the UV-Vis and fluorescent properties of 1a in non-polar solvents. Similar to 1a, we have also screened the UV-Vis and fluorescence properties of the other thiopyridines (1b–1p, see ESI†), however their results were not encouraging compared to 1a.![(Top) UV-Vis spectra of compound 1a in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 1a in different solvents [10−5 M; 25 °C; λex = (a) 343 nm, (b) 351 nm, (c) 344 nm, (d) 343 nm; slit = 2/2].](/image/article/2012/RA/c2ra21385k/c2ra21385k-f4.gif) | ||
| Fig. 4 (Top) UV-Vis spectra of compound 1a in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 1a in different solvents [10−5 M; 25 °C; λex = (a) 343 nm, (b) 351 nm, (c) 344 nm, (d) 343 nm; slit = 2/2]. | ||
| Compound | Solvent | λ abs max (nm) | λ em max (nm) | Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/char_e0d5.gif) (cm−1) (cm−1) |
Øaf |
|---|---|---|---|---|---|
a Quantum yield was calculated with respect to quinine sulphate dihydrate in water. Øf = quantum yield, λabsmax = absorbance maxima, λemmax = fluorescence maxima, Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/char_e0d5.gif) = Stoke's shift. = Stoke's shift.
|
|||||
| 1a | DMSO | 351 | 406 | 3860 | 0.09 |
| CH3CN | 343 | 421 | 5402 | 0.06 | |
| MeOH | 344 | 388 | 3297 | 0.10 | |
| CHCl3 | 343 | 426 | 5681 | 0.18 | |
Next, we were interested to study the UV-Vis and fluorescence properties of compounds (5a–d) and among them compounds 5a and 5d showed prominent quantum yields (see ESI† for 5b and 5c) in CHCl3. The UV-Vis and fluorescence data for compound 5a and 5d are summarized in Table 7 and Fig. 5 and 6 respectively.
![(Top) UV-Vis spectra of compound 5a in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 5a in different solvents [10−5 M; 25 °C; λex = (a) 329 nm, (b) 320 nm, (c) 306 nm, (d) 322 nm; slit = 2/2].](/image/article/2012/RA/c2ra21385k/c2ra21385k-f5.gif) | ||
| Fig. 5 (Top) UV-Vis spectra of compound 5a in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 5a in different solvents [10−5 M; 25 °C; λex = (a) 329 nm, (b) 320 nm, (c) 306 nm, (d) 322 nm; slit = 2/2]. | ||
![(Top) UV-Vis spectra of compound 5d in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 5d in different solvents [10−5M; 25 °C; λex = (a) 335 nm, (b) 342 nm, (c) 342 nm, (d) 341 nm; slit = 2/2].](/image/article/2012/RA/c2ra21385k/c2ra21385k-f6.gif) | ||
| Fig. 6 (Top) UV-Vis spectra of compound 5d in different solvents (10−5 M; 25 °C). (Bottom) Fluorescence spectra of compound 5d in different solvents [10−5M; 25 °C; λex = (a) 335 nm, (b) 342 nm, (c) 342 nm, (d) 341 nm; slit = 2/2]. | ||
| Compound | Solvent | λ abs max (nm) | λ em max (nm) | Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/char_e0d5.gif) (cm−1) (cm−1) |
Øaf |
|---|---|---|---|---|---|
a Quantum yield was calculated with respect to quinine sulphate dihydrate in water. Øf = Quantum yield, λabsmax = absorbance maxima, λemmax = fluorescence maxima, Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/char_e0d5.gif) = Stoke's shift. = Stoke's shift.
|
|||||
| 5a | DMSO | 329 | 394 | 5015 | 0.06 |
| CH3CN | 320 | 394 | 5870 | 0.08 | |
| MeOH | 322 | 367 | 3808 | 0.12 | |
| CHCl3 | 306 | 424 | 9095 | 0.14 | |
| 5d | DMSO | 342 | 504 | 9399 | 0.35 |
| CH3CN | 342 | 507 | 9516 | 0.23 | |
| MeOH | 335 | 377 | 3326 | 0.08 | |
| CHCl3 | 341 | 488 | 8834 | 0.57 | |
From these graphs we have found that in UV-Vis spectra, peaks appeared around 343–351 nm for 1a, 306–329 nm for 5a and 335–342 nm for 5d. In the case of compound 5a, a prominent blue-shift was observed in the chloroform solvent at 306 nm. The emission spectra appeared around 388–426 nm for 1a, 367–424 nm for 5a and 377–507 nm for 5d. Interestingly, for 5d a large red-shifted, low energy band was observed in both acetonitrile and dipolar aprotic DMSO solvent at 507 nm and 504 nm respectively. Also, for 5d in acetonitrile a high stoke shift Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/char_e0d5.gif) (9516 cm−1) was observed. The absorption maxima, emission maxima and fluorescence quantum yield depends on various factors such as the structure of the molecule, nature of the solvent, probe–probe interaction, probe–solvent interaction, temperature, pH and concentration etc.36c In this investigation we have observed that fluorescence quantum yield of these compounds is dependent on the type of the substituent group. Among all the compounds of Table 3, 1a showed a maximum quantum yield of 0.18 in CHCl3. The quantum yields of other compounds (1b–1p) were in the range Øf = 0.00–0.05. Substitution effects have been greatly observed in the case of 1a, which may be due to the presence of 4-OMe, a strong electron donating group in the C4-aryl of the pyridine ring as compared to the other derivatives. We believe that this –OMe enables the effective charge transfer in the excited state and reduces the non-radiative decay which causes intense fluorescence. Similarly, 5d shows a maximum quantum yield of Øf = 0.57 in CHCl3 among all the synthesized pyridine derivatives. This may be due to the presence of a secondary nitrogen atom in the 4-position of the piperazine moiety that has an influence on the tertiary nitrogen atom at the C6 position of the pyridine ring, likely via a through-space interaction. From these studies we have concluded that for these types of highly substituted pyridines, the photophysical behaviour is dependent on the substitution pattern as well as the types of solvents used i.e. the surrounding environment of the molecule. Considering the above behaviours, we believe that these substituted pyridines may find potential application as new fluorescent probes or luminescence material.
(9516 cm−1) was observed. The absorption maxima, emission maxima and fluorescence quantum yield depends on various factors such as the structure of the molecule, nature of the solvent, probe–probe interaction, probe–solvent interaction, temperature, pH and concentration etc.36c In this investigation we have observed that fluorescence quantum yield of these compounds is dependent on the type of the substituent group. Among all the compounds of Table 3, 1a showed a maximum quantum yield of 0.18 in CHCl3. The quantum yields of other compounds (1b–1p) were in the range Øf = 0.00–0.05. Substitution effects have been greatly observed in the case of 1a, which may be due to the presence of 4-OMe, a strong electron donating group in the C4-aryl of the pyridine ring as compared to the other derivatives. We believe that this –OMe enables the effective charge transfer in the excited state and reduces the non-radiative decay which causes intense fluorescence. Similarly, 5d shows a maximum quantum yield of Øf = 0.57 in CHCl3 among all the synthesized pyridine derivatives. This may be due to the presence of a secondary nitrogen atom in the 4-position of the piperazine moiety that has an influence on the tertiary nitrogen atom at the C6 position of the pyridine ring, likely via a through-space interaction. From these studies we have concluded that for these types of highly substituted pyridines, the photophysical behaviour is dependent on the substitution pattern as well as the types of solvents used i.e. the surrounding environment of the molecule. Considering the above behaviours, we believe that these substituted pyridines may find potential application as new fluorescent probes or luminescence material.
Conclusion
In summary, the present work describes an efficient one-pot multicomponent strategy for the synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines from the reaction of aldehydes, malononitrile and thiols using a cost effective, environmentally benign KOH as the catalyst. This protocol has the advantages of a wide scope of substrates, operational simplicity, no need of column chromatographic separation, shorter reaction times, high yields, ready availability and low cost of the catalyst. To the best of our knowledge we have for the first time studied the photophysical properties of 2-amino-3,5-dicarbonyl-6-sulfanylpyridines and have compared with the analogues of substituted amino and oxo-pyridines.Experimental
General Information
All reagents and chemicals required for the reaction were procured from commercial sources and used without further purification. IR spectra were recorded in a Shimadzu FTIR spectrophotometer. 1H NMR spectra and 13C NMR spectra were recorded on a Jeol 500, Varian 400 and Bruker 300/400/500 MHz spectrometer in CDCl3 and DMSO-d6 using TMS as the internal reference. Elemental analyses were carried out in a Perkin Elmer 2400 automatic CHN analyzer or Elementer Vario EL III. All new compounds were characterized by recording melting point without correction, 1H NMR, 13C NMR and elemental analysis. The UV-Vis absorption spectra were recorded on Shimadzu UV-Vis spectrophotometer (UV-2550) and fluorescence spectra was recorded on a Horiba Jobin Yuon fluoromax-4 spectrofluorometer. Quantum yields were determined with respect to quinine sulphate as a standard.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After completion of the reaction, the reaction mixture was gradually cooled to room temperature. The solid product was collected by simple filtration and washed with ethanol and dried. The solid was dissolved in ethyl acetate and washed with water. The collected ethyl acetate layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure to get the crude solid which was then purified by recrystalization from ethanol (for the product: 1b–1e, 1g, 1j,1k and 1n,1o; Table 1) and acetonitrile (for the product: 1a, 1f, 1h–1i, 1l,1m and 1p; Table 1) to obtain pure products.
2). After completion of the reaction, the reaction mixture was gradually cooled to room temperature. The solid product was collected by simple filtration and washed with ethanol and dried. The solid was dissolved in ethyl acetate and washed with water. The collected ethyl acetate layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure to get the crude solid which was then purified by recrystalization from ethanol (for the product: 1b–1e, 1g, 1j,1k and 1n,1o; Table 1) and acetonitrile (for the product: 1a, 1f, 1h–1i, 1l,1m and 1p; Table 1) to obtain pure products.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After the completion of the reaction the solid precipitated out at the stipulated time mentioned in Table 4. The solid precipitate was filtered off to obtain the desired product. The crude product was then recrystalized from acetonitrile.
2). After the completion of the reaction the solid precipitated out at the stipulated time mentioned in Table 4. The solid precipitate was filtered off to obtain the desired product. The crude product was then recrystalized from acetonitrile.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). After the completion of the reaction the solid precipitate came out at the stipulated time mentioned in Table 4. The solid precipitate was filtered off to obtain the desired product. The crude product was purified by recrystalization from acetonitrile.
5). After the completion of the reaction the solid precipitate came out at the stipulated time mentioned in Table 4. The solid precipitate was filtered off to obtain the desired product. The crude product was purified by recrystalization from acetonitrile.
Acknowledgements
We gratefully acknowledge financial support from the Department of Science and Technology India, with Sanction No. SR/FT/CS-042/2009 for carrying out this work. M.N.K and S.P are thankful to CSIR and UGC respectively for their research fellowships. The authors are grateful to SAIF CDRI Lucknow, SAIF IIT Madras and IIT Guwahati for providing analytical facilities. Authors are also thankful to IIT Patna for providing basic facilities to carry out this work. We thankfully acknowledge Dr. Debabrata Seth for his valuable discussion on photophysical properties.References
- (a) J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) I. Ugi, A. Domling and W. Horl, Endeavour, 1994, 18, 115–122 CrossRef CAS; (c) H. Bienayme, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321–3329 CrossRef CAS; (d) R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123–131 CrossRef CAS; (e) A. Domling, Curr. Opin. Chem. Biol., 2000, 4, 318–323 CrossRef CAS; (f) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef CAS; (g) A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS.
- (a) S. L. Schreiber, Science, 2000, 287, 1964–1969 CrossRef CAS; (b) M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46–58 CrossRef; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51–80 CAS; (d) S. L. Schreiber, Chem. Eng. News, 2000, 81, 51–61 Search PubMed.
- (a) R. V. A. Orru and E. Ruijter, Synthesis of Heterocycles via Multicomponent Reactions I & II (Topics in Heterocyclic Chemistry), Springer, 2010 Search PubMed; (b) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899–907 CrossRef CAS; (c) J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300–1308 CrossRef CAS.
- M. Schwoerer, H. C. Volf, Wiley-VCH, Weinheim, 2005.
- L. Jayasinghe, C. P. Jayasooriya, N. Hara and Y. Fujimoto, Tetrahedron Lett., 2003, 44, 8769–8771 CrossRef CAS.
- G. Bringmann, Y. Reichert and V. V. Kane, Tetrahedron, 2004, 60, 3539–3574 CrossRef CAS.
- (a) S. Tu, R. Jia, B. Jiang, J. Zhang, Y. Zhang, C. Yao and S. Ji, Tetrahedron, 2007, 63, 381–388 CrossRef CAS; (b) F. Krohnke, Synthesis, 1976, 1–24 CrossRef; (c) F. Neve, A. Crispini and S. Campagna, Inorg. Chem., 1997, 36, 6150–6256 CrossRef CAS; (d) L. R. MacGillivray, P. R. Diamente, J. L. Reid and J. A. Ripmeester, Chem. Commun., 2000, 359–360 RSC.
- B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson, K. N. Klotz and J. Linden, Pharmacol. Rev., 2001, 53, 527–552 CAS.
- M. Z. Chen and G. C. Micalizio, J. Am. Chem. Soc., 2012, 134, 1352–1356 CrossRef CAS.
- M. Ohashi, I. Takeda, M. Ikawa and S. Ogoshi, J. Am. Chem. Soc., 2011, 133, 18018–18021 CrossRef CAS.
- Y. S. Chun, J. H. Lee, J. H. Kim, Y. O. Ko and S. Lee, Org. Lett., 2011, 13, 6390–6393 CrossRef CAS.
- Z. H. Ren, Z. Y. Zhang, B. Q. Yang, Y. Y. Wang and Z. H. Guan, Org. Lett., 2011, 13, 5394–5397 CrossRef CAS.
- Y. Zou, Q. Liu and A. Deiters, Org. Lett., 2011, 13, 4352–4355 CrossRef CAS.
- N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Y. Antipin, I. V. Magedov and A. Kornienko, J. Org. Chem., 2007, 72, 3443–3453 CrossRef CAS.
- S. K. Srivastava, R. P. Tripathi and R. Ramachandran, J. Biol. Chem., 2005, 280, 30273–30281 CrossRef CAS.
- M. A. Azuine, H. Tokuda, J. Takayasu, F. Enjyo, T. Mukainaka, T. Konoshima, H. Nishino and G. J. Kapadia, Pharmacol. Res., 2004, 49, 161–169 Search PubMed.
- H. Harada, S. Watanuki, T. Takuwa, K. Kawaguchi, T. Okazaki, Y. Hirano, C. Saitoh, PCT Int. Appl., WO 2002006237 A1 20020124, 2002 Search PubMed.
- H. Chen, W. Zhang, R. Tam, A. K. Raney, PCT Int. Appl., WO 2005058315 A1 20050630, 2005 Search PubMed.
- (a) J. M. Quintela, C. Peinador, M. C. Veiga, L. M. Botana, A. Alfonso and R. Riguera, Eur. J. Med. Chem., 1998, 33, 887–897 CrossRef CAS; (b) A. M. E. Attia, A. El-H. and A. A. Ismail, Tetrahedron, 2003, 59, 1749–1752 Search PubMed; (c) L. C. W. Chang, J. K. von Frijtag Drabbe Kunzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2005, 48, 2045–2053 CrossRef CAS.
- N. M. Evdokimov, I. V. Magedov and A. S. Kireev, Org. Lett., 2006, 8, 899–902 CrossRef CAS.
- B. C Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 72, 3152–3154 CrossRef CAS.
- K. Guo, R. Mutter, W. Heal, T. R. K. Reddy, H. Cope, S. Pratt, M. J. Thompson and B. Chen, Eur. J. Med. Chem., 2008, 43, 93–106 Search PubMed.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. K. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897–3900 CrossRef CAS.
- S. Banerjee and G. Sereda, Tetrahedron Lett., 2009, 50, 6959–6962 CrossRef CAS.
- K. N. Singh and S. K. Singh, ARKIVOC, 2009, xiii, 153–160.
- R. Mamgain, R. Singh and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69–73 Search PubMed.
- P. V. Shinde, S. S. Sonar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2010, 51, 1309–1312 CrossRef CAS.
- M. R. P. Heravi and F. Fakhr, Tetrahedron Lett., 2011, 52, 6779–6782 CrossRef.
- M. L. Kantam, K. Mahendar and S. Bhargava, J. Chem. Sci., 2010, 122, 63–69 CrossRef CAS.
- M. Thimmaiah, P. Li, S. Regati, B. Chen and J. C-G. Zhao, Tetrahedron Lett., 2012, 53, 4870–4872 Search PubMed.
- (a) L. H. Choudhury and T. Parvin, Tetrahedron, 2011, 67, 8213–8228 CrossRef CAS; (b) S. Pal, L.H. Choudhury and T. Parvin, Mol. Diversity, 2012, 16, 129–143 Search PubMed.
- X. Xin, Y. Wang, S. Kumar, X. Liu, Y. Lin and D. Dong, Org. Biomol. Chem., 2010, 8, 3078–3082 RSC.
- T. R. K. Reddy, R. Mutter, W. Heal, K. Guo, V. J. Gillet, S. Pratt and B. Chen, J. Med. Chem., 2006, 49, 607–615 CrossRef CAS.
- A. S. Alvarez-Insua, M. Lora-Tamayo and J. L. Soto, J. Heterocycl. Chem., 1970, 7, 1305–1309 Search PubMed.
- (a) Y. Araki, A. Andoh, Y. Fujiyama, K. Hata, J. Makino, T. Okuno, F. Nakanura and T. Bamba, J. Chromatogr., Biomed. Appl., 2001, 753, 209–215 CrossRef CAS; (b) M. D. Bowman, M. M. Jacobson and H. E. Blackwell, Org. Lett., 2006, 8, 1645–1648 CrossRef CAS.
- (a) W. T. Mason, Fluorescent and Luminescent Probes for Biological Activity, Second Edition, Academic Press, 1999 Search PubMed; (b) M. S. T. Goncalves, Chem. Rev., 2009, 109, 190–212 CrossRef CAS; (c) J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 2006, 3rd edn, Springer Search PubMed; (d) M. Moller and A. Denicola, Biochem. Mol. Biol. Educ., 2002, 30, 309–312 CrossRef; (e) M. L Capobianco, G. Barbarella and A. Manetto, Molecules, 2012, 17, 910–933 Search PubMed; (f) H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS; (g) V. S. Padalkar and N. Sekar, Curr. Chem. Lett., 2012, 1, 1–12 Search PubMed.
- (a) M. Becuwe, D. Landy, F. Delattre, F. Cazier and S. Fourmentin, Sensors, 2008, 8, 3689–3705 CrossRef CAS; (b) M. T. Sharbati and F. Emami, Opt. Express, 2011, 19, 3619–3626 Search PubMed; (c) Y. Sun, L. Duan, D. Zhang, J. Qiao, G. Dong, L. Wang and Y. Qiu, Adv. Funct. Mater., 2011, 21, 1881–1886 CrossRef CAS; (d) V. Raghukumar, D. Thirumalai, V. T. Ramakrishnan, V. Karunakara and P. Ramamurthy, Tetrahedron, 2003, 59, 3761–3768 CrossRef CAS; (e) S. W. Chiu, L.-Y. Lin, H.-W. Lin, Y.-H. Chen, Z.-Y. Huang, Y.-T. Lin, F. Lin, Y.-H. Liu and K.-T. Wong, Chem. Commun., 2012, 48, 1857–1859 RSC.
- (a) M. Schwoerer and H. C. Wolf, Organic Molecular Solids, 1, 2007, John Wiley & Sons Search PubMed; (b) A. Aviram and M. A. Ratner, Chem. Phys. Lett., 1974, 29, 277–283 CrossRef CAS; (c) C. Joachim, J. K. Gimzewski and A. Aviram, Nature, 2000, 408, 541–548 CrossRef CAS; (d) I. Perez-D, J. Hihath, Y. Lee, L. Yu, L. Adamska, M. A. Kozhushner, I. I. Oleynik and N. Tao, Nat. Chem., 2009, 1, 635–641 CrossRef CAS; (e) E. Kelderman, W. Verboom, J. F. J. Engbersen, S. Harkema, G. J. T. Heesink, E. Lehmusvaara, N. F. V. Hulst and D. N. Reinhoudt, Chem. Mater., 1992, 4, 626–631 Search PubMed.
- J. N. Demas and G. A. Crosby, J. Phys. Chem., 1971, 75, 991–1024 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C-NMR spectra of all synthesized compounds. CCDC reference numbers 875077. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21385k |
| This journal is © The Royal Society of Chemistry 2012 |




