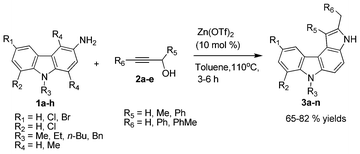
Zinc triflate catalyzed regioselective synthesis of pyrrolo[2,3-c]carbazoles via heteroannulation†
Mayavan
Viji
and
Rajagopal
Nagarajan
*
School of Chemistry, University of Hyderabad, Hyderabad 500 046, India. E-mail: rnsc@uohyd.ernet.in; Fax: +91 40 66794831
First published on 6th September 2012
Abstract
We explored the zinc triflate catalyzed heteroannulation reaction of 3-aminocarbazoles with substituted propargyl alcohols to form pyrrolo[2,3-c]carbazoles in moderate to good yields. This transformation proceeds with good regioselectivity and without the addition of additives or ligands.
Introduction
Great attention has been devoted to nitrogen-containing heterocycles, because they are found in a wide range of biologically important molecules and alkaloids.1–4 The carbazole skeleton is generally recognized as an important structure in medicinal chemistry,1 and in particular, heteroannulated carbazole alkaloids are abundantly found in numerous natural products, play an important role in pharmaceuticals, and a substantial number of their applications have been developed at the laboratory and industrial scale. Interest in this area is evidenced by the large number of papers and reviews appearing in the literature over the past several years.2 Recently, significant research effort has been focused on the preparation of pyrrolocarbazole scaffolds due to their promising biological activity, including antidepressant, anticancer, and antibacterial activities.3 Dictyodendrins A–E, pyrrolo[2,3-c]carbazole alkaloids, were isolated from dictyodendrilla verongiformis, showing 100% telomerase inhibitory activity at 50 μg mL−1 (Fig. 1).4 | ||
| Fig. 1 Structure of some of the Dictyodendrin alkaloids. | ||
Heteroannulation reactions have gained significant importance in organic chemistry. They have been proven to be valuable methods, and vast numbers of heterocyclic molecules have been synthesized. Accordingly, it is not surprising that there are several reports in the literature for the synthesis of heterocyclic compounds using this heteroannulation reaction.5
In the last few decades, much effort has been devoted to developing transition metal catalyzed reactions for the facile construction of the skeleton of heterocycles, in particular Lewis acid catalyzed annulation processes.6 In recent years zinc triflate has been employed as catalyst in a wide range of organic syntheses.7 We have recently reported an efficient general route for the synthesis of RuCl3/SnCl2 mediated pyrrolo[2,3-c]carbazoles in good yield.3d In this context, we wish to continue to develop efficient methods for the synthesis of this class of compounds, especially routes based upon easily prepared starting materials.
Herein, we report a highly efficient method for the regioselective synthesis of pyrrolo[2,3-c]carbazole scaffolds via a zinc triflate promoted heteroannulation reaction (Scheme 1). The reaction is catalyzed by 10 mol% Zn(OTf)2, using which it is possible to couple 3-aminocarbazole with propargyl alcohol in good yield.
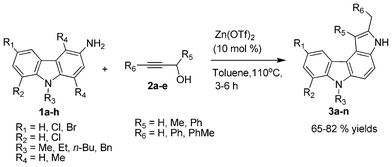 | ||
| Scheme 1 Schematic representation of present work. | ||
Results and discussion
Substituted 3-aminocarbazoles8a–c and propargyl alcohol8d–f derivatives were prepared based on the reported literature. The reaction of 9-ethyl-3-aminocarbazole (1a) with propargyl alcohol (2a) was selected to optimize the experimental conditions for the heteroannulation reaction, and the results are summarized in Table 1. The reaction afforded the desired product, pyrrolo[2,3-c]carbazole 3a, with 35% yield when using dioxane as the solvent, 5 mol% Zn(OTf)2 as the catalyst, and conventional heating at 110 °C (Table 1, entry 1). Encouraged by this result in our initial study, we systematically evaluated a broad range of reaction conditions, namely the effects of altering the catalyst, ligand, solvent, temperature, and reaction time on the synthesis of 3a, and the results are summarized in Table 1. The two parameters found to have the most significant impact on the cyclization were the choice of solvent and catalyst and the results are presented in Table 1.| Entry | Catalyst | Solvent | Time (h) | Temp. (°C) | Yieldg (%) |
|---|---|---|---|---|---|
| a Unless otherwise indicated, all the reactions were conducted in a RB using 9-ethyl-3-aminocarbazole 1a (1 equiv.), propargyl alcohol 2a (1.5 equiv.) catalyst (10 mol%), and 10 mL solvent. b 5 mol% catalyst used. c Reaction conducted in a pressure tube with 3 mL solvent. d 1.0 equiv. of 2a was used. e 15 mol% catalyst used. f Toluene or dioxane. g Isolated yields. | |||||
| 1b | Zn(OTf)2 | Dioxane | 6 | 110 | 35 |
| 2 | La(OTf)3 | Toluene | 24 | 110 | 25 |
| 3 | In(OTf)3 | Toluene | 8 | 110 | 71 |
| 4 | InCl3 | Toluene | 20 | 110 | 61 |
| 5c | InCl3 | Toluene | 8 | 130 | 30 |
| 6 | La(OTf)3 | Toluene | 8 | 110 | 21 |
| 7c | Zn(OTf)2 | CH3CN | 8 | 100 | 15 |
| 8 | Zn(OTf)2 | CH3CN | 8 | 100 | 62 |
| 9d | Zn(OTf)2 | Toluene | 4 | 110 | 45 |
| 10 | Zn(OTf)2 | Toluene | 4 | 110 | 75 |
| 11e | Zn(OTf)2 | Toluene | 10 | 120 | 75 |
| 12 | Zn(OTf)2 | Toluene | 10 | 70 | 32 |
| 13 | CAN | Toluene | 12 | 110 | 8 |
| 14 | InCl3 | Dioxane | 12 | 110 | 43 |
| 15 | InCl3 | THF | 10 | 80 | — |
| 16 | Cu(OTf)2 | Solventf | 24 | 110 | — |
| 17 | Ag(OTf) | Solventf | 24 | 110 | — |
| 18 | Sc(OTf)3 | Toluene | 10 | 110 | 35 |
| 19 | Zn(OAc)2 | Toluene | 8 | 110 | 12 |
| 20 | ZnCl2 | Toluene | 8 | 110 | 47 |
| 21 | Zn(NO3)2·6H2O | Toluene | 8 | 110 | — |
| 22 | ZnBr2 | Toluene | 8 | 110 | 26 |
| 23 | ZnCO3 | Toluene | 12 | 110 | — |
| 24 | Zn(OTf)2 | THF | 12 | 80 | 47 |
| 25 | La(OTf)3 | DMA | 8 | 120 | — |
| 26 | p-TSA | Toluene | 8 | 110 | 16 |
| 27 | Zn(OTf)2 | DMF | 8 | 110 | — |
| 28 | — | Toluene | 24 | 120 | — |
We first examined the influence of triflates. Different triflate sources were examined, and 10 mol% Zn(OTf)2 showed the highest activity, followed by In(OTf)3 (10 mol%) (Table 1, entries 10 and 3). However, catalysts like La(OTf)3 and Sc(OTf)3 did not facilitate good conversion, and relatively low yields were obtained (Table 1, entries 2, 6 and 18). Catalysts such as Cu(OTf)2 and Ag(OTf) did not produce the above mentioned transformation. When conducted at a moderate temperature of 70 °C with 10 mol% zinc triflate as the catalyst, the reaction proceeded with a lower yield, whereas when it was conducted at higher reaction temperature of 120 °C, the yield did not increase beyond 75% (entries 11–12, Table 1). The screening experiments also showed that increasing the amount of Zn(OTf)2 did not enhance the yield, and even prolonged the reaction time to 10 h. Among the other catalysts, InCl3 promoted the desired annulated product 3a in moderate yield (entry 4).
Of the various zinc salts that were tested in this reaction, zinc triflate gave the best yield when the reaction was conducted in toluene (Table 1, entries 10 and 19–23). A lower yield was obtained when the reaction was carried out with ZnCl2 as the catalyst (Table 1, entry 20). Control experiments showed that omitting the catalyst did not form the product (entry 28). After a comprehensive screening, we found that zinc triflate was superior among all the other catalysts that were examined in this reaction, with a high level of regioselectivity. In(OTf)3 and InCl3 also gave good yields, but zinc triflate gave a somewhat better yield than these catalysts. Moreover, zinc triflate is a commercially cheaper catalyst, and is easier to handle than the other lanthanide triflates and indium catalysts, which require an inert atmosphere for this heteroannulation reaction.
Further inspection of the reaction conditions reveals that the reaction proceeded efficiently in solvents such as CH3CN, THF, and 1,4-dioxane, although they were less efficient compared with toluene. We also checked the effect of the ligands on the reaction yield, and found that they did not play a significant role in this reaction. Various ligands such as dppe, PPh3, dppf, P(OEt)3, and PCy3 were all unimpressive. The use of 1.5 equiv. of 2a rather than 1.0 equiv. improves the yield of the reaction (entry 9, Table 1). The best result was obtained when the reaction was conducted by mixing 1a, 2a and catalyst zinc triflate (10 mol%) successively in toluene, and then refluxing at 110 °C for 4 h to give the desired product 3a in 75% yield (entry 10).
Having identified these optimal conditions, we sought to examine the scope and the generality of the method by applying it to a range of substituted 3-aminocarbazoles and propargyl alcohols, and the results are shown in Table 2. The products 3a–n were generated from 1a–h and 2a–e in moderate to good yields. Notably, regioselectivity was observed in this transformation. All the products displayed spectroscopic data in agreement with the expected pyrrolocarbazoles, and the structures of 3f and 3k were further confirmed by X-ray data.9 From the ORTEP (Fig. 2) we conclude that the methyl group in the product comes from the R6 position of the propargyl alcohol. If R6 = H, the products have a methyl group at the C2 position (which is near to the nitrogen atom), and if R6 = Ph, the products have a benzyl group at the C2 position. The pure products were easily obtained by column chromatography over alumina.
 | ||
| Fig. 2 ORTEP of 3f and 3k. | ||
 | ||
| Scheme 2 Possible mechanism of the present reaction. | ||
|
|
|||
|---|---|---|---|
| a All the reactions were conducted in a RB using 3-aminocarbazole 1a–n (1 equiv.), propargyl alcohol 2a–e (1.5 equiv.), zinc triflate (10 mol%), and 20 mL of toluene stirred 110 °C. | |||

|

|

|
|

|

|

|
|

|

|

|

|
|

|
||
The reaction mechanism (Scheme 2) was proposed on the basis of a literature report.7a The reaction mechanism consists of the following steps: in the first step, hydroamination of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond of the propargyl alcohol takes place. Zinc triflate makes the alkyne carbon electron deficient, thereby facilitating the attack of the nitrogen lone pair on the alkyne carbon, followed by the hydrogen migration which gives rise to the aminoketone structure IA. The migration of the nitrogen lone pair gives rise to structure IIA, and then rearomatization takes place in the carbazole ring, resulting in the structure IIIA. Finally, removal of a water molecule gives the desired product 3a.
C triple bond of the propargyl alcohol takes place. Zinc triflate makes the alkyne carbon electron deficient, thereby facilitating the attack of the nitrogen lone pair on the alkyne carbon, followed by the hydrogen migration which gives rise to the aminoketone structure IA. The migration of the nitrogen lone pair gives rise to structure IIA, and then rearomatization takes place in the carbazole ring, resulting in the structure IIIA. Finally, removal of a water molecule gives the desired product 3a.
Conclusion
We have established a general method for the preparation of fused pyrrolo[2,3-c]- and pyrrolo[2,3-b]carbazole derivatives from 3-aminocarbazoles and propargyl alcohol derivatives via a zinc triflate catalyzed heteroannulation reaction.Experimental section
General procedure
All 1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer, operating at 400 and 100 MHz respectively. Chemical shifts for 1H NMR are expressed in parts per million (ppm) relative to tetramethylsilane (δ = 0.00 ppm). Chemical shifts for 13C NMR are expressed in ppm relative to CDCl3 (δ =77.0 ppm). Multiplicities are indicated as follows (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and coupling constants are given in Hz. Chemical shifts of common trace 1H NMR impurities (CDCl3, ppm): H2O, 1.56; EtOAc, 1.26, 2.05, 4.12; CH2Cl2, 5.30; CDCl3, 7.26. IR spectra were recorded on a JASCO FT/IR-5300 spectrometer; absorptions are reported in cm−1. Mass spectra were recorded on either a VG7070H mass spectrometer using an EI technique, or a Shimadzu-LCMS-2010A mass spectrometer. Elemental analyses (CHN) were recorded on a Thermo Finnigan Flash EA 1112 analyzer at the School of Chemistry, University of Hyderabad. Routine monitoring of the reactions was performed by TLC, using silica gel plates (Merck 60 F254). Compounds were visualized with a UV light at 254 nm. Further visualization was achieved by staining with iodine. Column chromatography was carried out employing neutral alumina. Commercially available reagents and solvents were purchased and used without further purification. Melting points were measured in open capillary tubes and are uncorrected.General procedure for preparation of pyrrolo[2,3-c]carbazoles
To a stirred solution of 9-ethyl-3-aminocarbazole 1a (1 equiv.) and propargyl alcohol 2a (1.5 equiv.) in toluene (20 mL) was added zinc triflate (10 mol%), and the reaction mixture was stirred at 110 °C for 4 h. After completion of the reaction, as indicated by TLC, the reaction mixture was filtered through a short celite bed. After the solvent was evaporated, the organic layer was extracted with dichloromethane (3 × 10 mL) and washed with water, dried over anhydrous Na2SO4 and evaporated to afford the crude product, which was separated by neutral alumina column chromatography, eluting with a hexane–ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) mixture. The solvent was evaporated to dryness to give the pure product 3a. The same procedure was followed for the preparation of all other products (3b–n).
5 v/v) mixture. The solvent was evaporated to dryness to give the pure product 3a. The same procedure was followed for the preparation of all other products (3b–n).
6-Ethyl-2-methyl-3,6-dihydropyrrolo[2,3-c]carbazole (3a)
Pale white colored solid; yield: 75%, mp: 42 °C, IR (KBr): 3402, 2974, 2916, 1728, 1662, 1595, 1481, 14229, 1329, 1228, 1018, 781, 734 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.60. 1H NMR (400 MHz, TMS, CDCl3): δ 8.26 (d, 1H, J = 7.6 Hz), 8.02 (s, 1H), 7.49–7.41 (m, 3H), 7.30–7.28 (m, 1H), 7.23–7.21 (m, 1H), 6.80 (s, 1H), 4.44 (q, 2H, J = 7.2 Hz), 2.57 (s, 3H), 1.45 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 135.1, 135.0, 130.5, 123.7, 123.2, 122.6, 121.3, 118.1, 113.2, 109.1, 108.1, 102.8, 99.2, 37.7, 14.06, 14.01. LC-MS: m/z = 249 (M + H)+, positive mode; Anal. calcd for molecular formula C17H16N2; C, 82.22; H, 6.49; N, 11.28%; found: C, 82.36; H, 6.55; N, 11.15%.
3); Rf = 0.60. 1H NMR (400 MHz, TMS, CDCl3): δ 8.26 (d, 1H, J = 7.6 Hz), 8.02 (s, 1H), 7.49–7.41 (m, 3H), 7.30–7.28 (m, 1H), 7.23–7.21 (m, 1H), 6.80 (s, 1H), 4.44 (q, 2H, J = 7.2 Hz), 2.57 (s, 3H), 1.45 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 135.1, 135.0, 130.5, 123.7, 123.2, 122.6, 121.3, 118.1, 113.2, 109.1, 108.1, 102.8, 99.2, 37.7, 14.06, 14.01. LC-MS: m/z = 249 (M + H)+, positive mode; Anal. calcd for molecular formula C17H16N2; C, 82.22; H, 6.49; N, 11.28%; found: C, 82.36; H, 6.55; N, 11.15%.
2,6-Dimethyl-3,6-dihydropyrrolo[2,3-c]carbazole (3b)
Brown colored semi-solid; yield: 70%, IR (KBr): 3449, 2924, 1626, 1547, 1448, 1271, 852, 817, 750, 626, 432 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.52. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 7.6 Hz), 8.06 (s, 1H), 7.44–7.41 (m, 3H), 7.28–7.26 (m, 1H), 7.20–7.18 (m, 1H), 6.78 (s, 1H), 3.90 (s, 3H), 2.56 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 140.1, 136.1, 135.2, 130.5, 123.7, 123.0, 122.4, 121.1, 118.2, 113.0, 109.1, 108.1, 102.7, 99.1, 29.4, 14.0. LC-MS: m/z = 235 (M + H)+, positive mode; Anal. calcd for molecular formula C16H14N2; C, 82.02; H, 6.02; N, 11.96%; found: C, 81.92 H, 6.12; N, 11.78%.
3); Rf = 0.52. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 7.6 Hz), 8.06 (s, 1H), 7.44–7.41 (m, 3H), 7.28–7.26 (m, 1H), 7.20–7.18 (m, 1H), 6.78 (s, 1H), 3.90 (s, 3H), 2.56 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 140.1, 136.1, 135.2, 130.5, 123.7, 123.0, 122.4, 121.1, 118.2, 113.0, 109.1, 108.1, 102.7, 99.1, 29.4, 14.0. LC-MS: m/z = 235 (M + H)+, positive mode; Anal. calcd for molecular formula C16H14N2; C, 82.02; H, 6.02; N, 11.96%; found: C, 81.92 H, 6.12; N, 11.78%.
6-Butyl-2-methyl-3,6-dihydropyrrolo[2,3-c]carbazole (3c)
Pale red colored semi-solid; yield: 72%, IR (KBr): 3398, 3051, 2957, 2930, 2872, 1593, 1547, 1477, 1429, 1367, 1329, 1284, 102, 734 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.62. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 7.6 Hz), 8.00 (s, 1H), 7.42–7.36 (m, 3H), 7.24–7.23 (m, 1H), 7.19–7.17 (m, 1H), 6.77(s, 1H), 4.34 (t, 2H, J = 7.2 Hz), 2.52 (s, 3H), 1.87–1.83 (m, 2H), 1.41–1.36 (m, 2H), 0.92 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.6, 135.5, 135.2, 130.4, 123.7, 123.1, 122.5, 121.2, 118.1, 113.0, 109.0, 108.4, 103.0, 99.1, 43.0, 31.4, 20.6, 14.0, 13.9. LC-MS: m/z = 277 (M + H)+, positive mode; Anal. calcd for molecular formula C19H20N2; C, 82.57; H, 7.29; N, 10.14%; found: C, 82.45; H, 7.23; N, 10.25%.
3); Rf = 0.62. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 7.6 Hz), 8.00 (s, 1H), 7.42–7.36 (m, 3H), 7.24–7.23 (m, 1H), 7.19–7.17 (m, 1H), 6.77(s, 1H), 4.34 (t, 2H, J = 7.2 Hz), 2.52 (s, 3H), 1.87–1.83 (m, 2H), 1.41–1.36 (m, 2H), 0.92 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.6, 135.5, 135.2, 130.4, 123.7, 123.1, 122.5, 121.2, 118.1, 113.0, 109.0, 108.4, 103.0, 99.1, 43.0, 31.4, 20.6, 14.0, 13.9. LC-MS: m/z = 277 (M + H)+, positive mode; Anal. calcd for molecular formula C19H20N2; C, 82.57; H, 7.29; N, 10.14%; found: C, 82.45; H, 7.23; N, 10.25%.
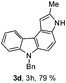
6-Benzyl-2-methyl-3,6-dihydropyrrolo[2,3-c]carbazole (3d)
Pale red colored solid; yield: 79%, mp: 82 °C, IR (KBr): 3393, 2924, 2854, 1712, 1599, 1427, 1361, 1259, 1159, 1024, 736 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.56. 1H NMR (400 MHz, TMS, CDCl3): δ 8.27 (d, 1H, J = 7.6 Hz), 8.05 (s, 1H), 7.39–7.37 (m, 2H), 7.35 (d, 1H, J = 8.4 Hz), 7.27–7.18 (m, 4H), 7.13–7.10 (m, 3H), 6.81 (s, 1H), 5.57 (s, 2H), 2.56 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 139.9, 137.8, 135.7, 135.4, 130.7, 128.6, 127.2, 126.3, 124.0, 123.3, 122.5, 121.2, 118.6, 113.3, 109.3, 108.6, 103.2, 99.1, 46.7, 14.0. LC-MS: m/z = 311 (M + H)+, positive mode; Anal. calcd for molecular formula C22H18N2; C, 85.13; H, 5.85; N, 9.03%; found: C, 84.91; H, 5.79; N, 9.12%.
3); Rf = 0.56. 1H NMR (400 MHz, TMS, CDCl3): δ 8.27 (d, 1H, J = 7.6 Hz), 8.05 (s, 1H), 7.39–7.37 (m, 2H), 7.35 (d, 1H, J = 8.4 Hz), 7.27–7.18 (m, 4H), 7.13–7.10 (m, 3H), 6.81 (s, 1H), 5.57 (s, 2H), 2.56 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 139.9, 137.8, 135.7, 135.4, 130.7, 128.6, 127.2, 126.3, 124.0, 123.3, 122.5, 121.2, 118.6, 113.3, 109.3, 108.6, 103.2, 99.1, 46.7, 14.0. LC-MS: m/z = 311 (M + H)+, positive mode; Anal. calcd for molecular formula C22H18N2; C, 85.13; H, 5.85; N, 9.03%; found: C, 84.91; H, 5.79; N, 9.12%.
7,9-Dichloro-2,6-dimethyl-3,6-dihydropyrrolo[2,3-c]carbazole (3e)
White colored solid; yield: 74%, mp: 184 °C, IR (KBr): 3449, 2924, 1626, 1547, 1448, 1271, 852, 817, 750, 626, 432 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.50. 1H NMR (400 MHz, TMS, CDCl3): δ 8.11 (s, 1H), 8.01 (d, 1H, J = 1.6 Hz), 7.44 (d, 1H, J = 8.4 Hz), 7.31 (d, 1H, J = 2.0 Hz), 7.13 (d, 1H, J = 8.8 Hz), 6.69 (s, 1H), 4.21 (s, 3H), 2.55 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 137.8, 135.9, 133.8, 130.7, 126.6, 125.0, 123.3, 121.9, 119.2, 115.9, 112.0, 110.7, 102.9, 99.0, 32.2, 14.0. LC-MS: m/z = 301 (M − H)−, 303 (M + 2H)− negative mode; Anal. calcd for molecular formula C16H12N2Cl2; C, 63.38; H, 3.99; N, 9.24%; found: C, 63.45; H, 3.91; N, 9.15%.
3); Rf = 0.50. 1H NMR (400 MHz, TMS, CDCl3): δ 8.11 (s, 1H), 8.01 (d, 1H, J = 1.6 Hz), 7.44 (d, 1H, J = 8.4 Hz), 7.31 (d, 1H, J = 2.0 Hz), 7.13 (d, 1H, J = 8.8 Hz), 6.69 (s, 1H), 4.21 (s, 3H), 2.55 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 137.8, 135.9, 133.8, 130.7, 126.6, 125.0, 123.3, 121.9, 119.2, 115.9, 112.0, 110.7, 102.9, 99.0, 32.2, 14.0. LC-MS: m/z = 301 (M − H)−, 303 (M + 2H)− negative mode; Anal. calcd for molecular formula C16H12N2Cl2; C, 63.38; H, 3.99; N, 9.24%; found: C, 63.45; H, 3.91; N, 9.15%.
7,9-Dichloro-6-ethyl-2-methyl-3,6-dihydropyrrolo[2,3-c]carbazole (3f)
White colored solid; yield: 80%, mp: 118 °C, IR (neat) = 3406, 2976, 2930, 1620, 1599, 1548, 1462, 1373, 1300, 1280, 1195, 1105 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.60. 1H NMR (400 MHz, TMS, CDCl3): δ 8.01 (d, 1H, J = 1.64 Hz), 7.93 (s, 1H), 7.35–7.33 (m, 1H), 7.32 (d, 1H, J = 1.68 Hz), 7.12 (d, 1H, J = 8.8 Hz), 6.65 (s, 1H), 4.72 (q, 2H, J = 6.8 Hz), 2.46 (s, 3H), 1.40 (t, 3H, J = 6.8 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 136.8, 135.9, 133.0, 130.7, 127.0, 125.0, 123.3, 122.0, 119.2, 115.2, 112.3, 110.8, 102.9, 99.0, 39.5, 15.8, 13.9. LC-MS: m/z = 317 (M + H)+, 318 (M + 2H)+ positive mode; Anal. calcd for molecular formula C17H14N2Cl2; C, 64.37; H, 4.45; N, 8.83%; found: C, 64.21; H, 4.51; N, 8.96%.
3); Rf = 0.60. 1H NMR (400 MHz, TMS, CDCl3): δ 8.01 (d, 1H, J = 1.64 Hz), 7.93 (s, 1H), 7.35–7.33 (m, 1H), 7.32 (d, 1H, J = 1.68 Hz), 7.12 (d, 1H, J = 8.8 Hz), 6.65 (s, 1H), 4.72 (q, 2H, J = 6.8 Hz), 2.46 (s, 3H), 1.40 (t, 3H, J = 6.8 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 136.8, 135.9, 133.0, 130.7, 127.0, 125.0, 123.3, 122.0, 119.2, 115.2, 112.3, 110.8, 102.9, 99.0, 39.5, 15.8, 13.9. LC-MS: m/z = 317 (M + H)+, 318 (M + 2H)+ positive mode; Anal. calcd for molecular formula C17H14N2Cl2; C, 64.37; H, 4.45; N, 8.83%; found: C, 64.21; H, 4.51; N, 8.96%.
9-Bromo-6-ethyl-2-methyl-3,6-dihydropyrrolo[2,3-c]carbazole (3g)
Brown colored semi-solid; yield: 72%, IR (KBr): 3406, 2894, 1606, 1536, 1452, 1301, 858, 827, 750, 620 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.52. 1H NMR (400 MHz, TMS, CDCl3): δ 8.31 (d, 1H, J = 1.6 Hz), 8.08 (s, 1H), 7.49–7.47 (m, 1H), 7.45–7.42 (m, 1H), 7.31–7.29 (m, 1H), 7.18–7.16 (m, 1H), 6.74 (s, 1H), 4.39 (q, 2H, J = 7.2 Hz), 2.56 (s, 3H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 137.7, 135.58, 135.52, 130.5, 126.3, 124.9, 123.7, 122.4, 112.3, 110.9, 109.9, 109.5, 102.7, 99.1, 37.9, 13.9. LC-MS: m/z = 325 (M − H)−, 327 (M + 2H)− negative mode; Anal. calcd for molecular formula C17H15N2Br; C, 62.40; H, 4.62; N, 8.56%; found: C, 62.21; H, 4.71; N, 8.45%.
3); Rf = 0.52. 1H NMR (400 MHz, TMS, CDCl3): δ 8.31 (d, 1H, J = 1.6 Hz), 8.08 (s, 1H), 7.49–7.47 (m, 1H), 7.45–7.42 (m, 1H), 7.31–7.29 (m, 1H), 7.18–7.16 (m, 1H), 6.74 (s, 1H), 4.39 (q, 2H, J = 7.2 Hz), 2.56 (s, 3H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 137.7, 135.58, 135.52, 130.5, 126.3, 124.9, 123.7, 122.4, 112.3, 110.9, 109.9, 109.5, 102.7, 99.1, 37.9, 13.9. LC-MS: m/z = 325 (M − H)−, 327 (M + 2H)− negative mode; Anal. calcd for molecular formula C17H15N2Br; C, 62.40; H, 4.62; N, 8.56%; found: C, 62.21; H, 4.71; N, 8.45%.
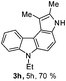
6-Ethyl-1,2-dimethyl-3,6-dihydropyrrolo[2,3-c]carbazole (3h)
Brown colored semi-solid; yield: 70%, IR (KBr): 3362, 3065, 2968, 2928, 1732, 1682, 1541, 1469, 1325, 1226, 804, 746 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.76. 1H NMR (400 MHz, TMS, CDCl3): δ 8.26 (s, 1H), 7.85–7.82 (m, 2H), 7.49 (d, 1H, J = 8.0 Hz), 7.44–7.40 (m, 2H), 7.23–7.22 (m, 1H), 4.35 (q, 2H, J = 7.2 Hz), 2.75 (s, 3H), 2.18 (s, 3H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 206.3, 169.4, 140.5, 137.6, 128.6, 126.3, 125.0, 123.1, 122.3, 120.8, 119.3, 118.2, 110.6, 109.0, 37.6, 31.8, 24.1, 13.8. LC-MS: m/z = 263 (M + H)+, positive mode; Anal. calcd for molecular formula C18H18N2; C, 82.41; H, 6.92; N, 10.68%; found: C, 82.25; H, 6.98; N, 10.58%.
3); Rf = 0.76. 1H NMR (400 MHz, TMS, CDCl3): δ 8.26 (s, 1H), 7.85–7.82 (m, 2H), 7.49 (d, 1H, J = 8.0 Hz), 7.44–7.40 (m, 2H), 7.23–7.22 (m, 1H), 4.35 (q, 2H, J = 7.2 Hz), 2.75 (s, 3H), 2.18 (s, 3H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 206.3, 169.4, 140.5, 137.6, 128.6, 126.3, 125.0, 123.1, 122.3, 120.8, 119.3, 118.2, 110.6, 109.0, 37.6, 31.8, 24.1, 13.8. LC-MS: m/z = 263 (M + H)+, positive mode; Anal. calcd for molecular formula C18H18N2; C, 82.41; H, 6.92; N, 10.68%; found: C, 82.25; H, 6.98; N, 10.58%.
6-Benzyl-1,2-dimethyl-3,6-dihydropyrrolo[2,3-c]carbazole (3i)
Brown colored semi-solid; yield: 65%, IR (KBr): 3398, 2918, 1636, 1607, 1438, 1263, 848, 817, 755, 626, 434 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.66. 1H NMR (400 MHz, TMS, CDCl3): δ 8.10 (d, 1H, J = 7.6 Hz), 7.95 (s, 1H), 7.68 (s, 1H), 7.38–7.33 (m, 2H), 7.26–7.27 (m, 1H), 7.43–7.16 (m, 6H), 5.55 (s, 2H), 2.42 (s, 3H), 2.25 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 137.8, 132.5, 131.4, 129.9, 129.2, 128.9, 128.8, 128.6, 127.1, 126.4, 126.3, 124.8, 123.6, 119.5, 118.0, 108.1, 100.4, 95.5, 46.6, 11.9, 8.7. LC-MS: m/z = 325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.02; H, 6.28; N, 8.56%.
3); Rf = 0.66. 1H NMR (400 MHz, TMS, CDCl3): δ 8.10 (d, 1H, J = 7.6 Hz), 7.95 (s, 1H), 7.68 (s, 1H), 7.38–7.33 (m, 2H), 7.26–7.27 (m, 1H), 7.43–7.16 (m, 6H), 5.55 (s, 2H), 2.42 (s, 3H), 2.25 (s, 3H). 13C NMR (100 MHz, TMS, CDCl3): δ 137.8, 132.5, 131.4, 129.9, 129.2, 128.9, 128.8, 128.6, 127.1, 126.4, 126.3, 124.8, 123.6, 119.5, 118.0, 108.1, 100.4, 95.5, 46.6, 11.9, 8.7. LC-MS: m/z = 325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.02; H, 6.28; N, 8.56%.
6-Ethyl-2-methyl-1-phenyl-3,6-dihydropyrrolo[2,3-c]carbazole (3j)
Brown colored semi-solid; yield: 78%, IR (KBr): 3456, 2992, 1613, 1587, 1458, 1280, 842, 813, 750, 686, 430 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.40. 1H NMR (400 MHz, TMS, CDCl3): δ 9.35 (s, 1H), 8.30 (d, 1H, J = 8.8 Hz), 7.97–7.95 (m, 2H), 7.90–7.88 (m, 1H), 7.58–7.47 (m, 6H), 7.24–7.22 (m, 1H), 4.42 (q, 2H, J = 7.2 Hz), 2.80 (s, 3H), 1.46 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 206.4, 165.9, 140.9, 137.4, 134.4, 131.9, 128.8, 127.2, 126.9, 126.3, 126.1, 122.7, 122.1, 120.9, 119.3, 118.7, 111.3, 109.1, 37.7, 31.9, 13.8. LC-MS: m/z =325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.26; H, 6.51; N, 8.81%.
3); Rf = 0.40. 1H NMR (400 MHz, TMS, CDCl3): δ 9.35 (s, 1H), 8.30 (d, 1H, J = 8.8 Hz), 7.97–7.95 (m, 2H), 7.90–7.88 (m, 1H), 7.58–7.47 (m, 6H), 7.24–7.22 (m, 1H), 4.42 (q, 2H, J = 7.2 Hz), 2.80 (s, 3H), 1.46 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 206.4, 165.9, 140.9, 137.4, 134.4, 131.9, 128.8, 127.2, 126.9, 126.3, 126.1, 122.7, 122.1, 120.9, 119.3, 118.7, 111.3, 109.1, 37.7, 31.9, 13.8. LC-MS: m/z =325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.26; H, 6.51; N, 8.81%.
2-Benzyl-6-ethyl-3,6-dihydropyrrolo[2,3-c]carbazole (3k)
Yellow colored solid; yield: 82%, mp: 132 °C, IR (neat) = 3402, 2974, 1604, 1483, 1431, 1381, 1332, 1230, 1151, 814, 734 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.66. 1H NMR (400 MHz, TMS, CDCl3): δ 8.24 (d, 1H, J = 7.6 Hz), 7.92 (s, 1H), 7.43–7.42 (m, 2H), 7.35–7.22 (m, 7H), 7.20–7.18 (m, 1H), 6.88 (s, 1H), 4.40 (q, 2H, J = 7.2 Hz), 4.23 (s, 2H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 138.8, 137.9, 135.0, 130.8, 128.9, 128.7, 126.7, 123.8, 123.2, 122.2, 121.3, 118.2, 113.3, 109.3, 108.2, 103.3, 99.8, 37.7, 34.9, 14.0. LC-MS: m/z = 325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.26; H, 6.15; N, 8.71%.
3); Rf = 0.66. 1H NMR (400 MHz, TMS, CDCl3): δ 8.24 (d, 1H, J = 7.6 Hz), 7.92 (s, 1H), 7.43–7.42 (m, 2H), 7.35–7.22 (m, 7H), 7.20–7.18 (m, 1H), 6.88 (s, 1H), 4.40 (q, 2H, J = 7.2 Hz), 4.23 (s, 2H), 1.41 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 138.8, 137.9, 135.0, 130.8, 128.9, 128.7, 126.7, 123.8, 123.2, 122.2, 121.3, 118.2, 113.3, 109.3, 108.2, 103.3, 99.8, 37.7, 34.9, 14.0. LC-MS: m/z = 325 (M + H)+, positive mode; Anal. calcd for molecular formula C23H20N2; C, 85.15; H, 6.21; N, 8.63%; found: C, 85.26; H, 6.15; N, 8.71%.
2-Benzyl-6-butyl-3,6-dihydropyrrolo[2,3-c]carbazole (3l)
Brown colored semi-solid; yield: 74%, IR (KBr): 3359, 2834, 1665, 1568, 1452, 1295, 859, 827, 762, 626, 458 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.74. 1H NMR (400 MHz, TMS, CDCl3): δ 8.16 (d, 1H, J = 7.6 Hz), 7.87 (s, 1H), 7.37–7.33 (3H, m), 7.27–7.21 (m, 4H), 7.16–7.11 (3H, m), 6.81 (s, 1H), 4.27 (t, 2H, J = 7.2 Hz), 4.17 (s, 2H), 1.78 (pent, 2H, J = 7.2 Hz), 1.34–1.28 (m, 2H), 0.84 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.6, 138.8, 137.9, 135.5, 130.7, 128.9, 128.7, 128.5, 128.3, 126.7, 123.7, 122.2, 121.2, 118.1, 109.2, 108.4, 103.5, 99.8, 43.0, 35.0, 31.4, 20.5, 13.9. LC-MS: m/z = 353 (M + H)+, positive mode; Anal. calcd for molecular formula C25H24N2; C, 85.19; H, 6.86; N, 7.95%; found: C, 85.21; H, 6.83; N, 7.76%.
3); Rf = 0.74. 1H NMR (400 MHz, TMS, CDCl3): δ 8.16 (d, 1H, J = 7.6 Hz), 7.87 (s, 1H), 7.37–7.33 (3H, m), 7.27–7.21 (m, 4H), 7.16–7.11 (3H, m), 6.81 (s, 1H), 4.27 (t, 2H, J = 7.2 Hz), 4.17 (s, 2H), 1.78 (pent, 2H, J = 7.2 Hz), 1.34–1.28 (m, 2H), 0.84 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.6, 138.8, 137.9, 135.5, 130.7, 128.9, 128.7, 128.5, 128.3, 126.7, 123.7, 122.2, 121.2, 118.1, 109.2, 108.4, 103.5, 99.8, 43.0, 35.0, 31.4, 20.5, 13.9. LC-MS: m/z = 353 (M + H)+, positive mode; Anal. calcd for molecular formula C25H24N2; C, 85.19; H, 6.86; N, 7.95%; found: C, 85.21; H, 6.83; N, 7.76%.
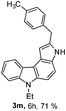
6-Ethyl-2-(4-methylbenzyl)-3,6-dihydropyrrolo[2,3-c]carbazole (3m)
Brown colored semi-solid; yield: 71%, IR (KBr): 3400, 3294, 2974, 2924, 2858, 1666, 1606, 1514, 1479, 1433, 1020, 734 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.68. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 8.0 Hz), 7.88 (s, 1H), 7.41 (d, 2H, J = 4 Hz), 7.28–7.24 (m, 2H), 7.16–7.09 (m, 5H), 6.84 (s, 1H), 4.36 (q, 2H, J = 7.2 Hz), 4.12 (s, 2H), 2.31 (s, 3H), 1.37 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 138.3, 136.2, 135.8, 135.0, 130.8, 129.4, 128.8, 123.8, 123.2, 122.3, 121.4, 118.2, 113.3, 109.4, 108.2, 103.2, 99.6, 37.7, 34.5, 21.1, 14.1. LC-MS: m/z = 339 (M + H)+, positive mode; Anal. calcd for molecular formula C24H22N2; C, 85.17; H, 6.55; N, 8.28%; found: C, 85.31; H, 6.45; N, 8.21%.
3); Rf = 0.68. 1H NMR (400 MHz, TMS, CDCl3): δ 8.23 (d, 1H, J = 8.0 Hz), 7.88 (s, 1H), 7.41 (d, 2H, J = 4 Hz), 7.28–7.24 (m, 2H), 7.16–7.09 (m, 5H), 6.84 (s, 1H), 4.36 (q, 2H, J = 7.2 Hz), 4.12 (s, 2H), 2.31 (s, 3H), 1.37 (t, 3H, J = 7.2 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 139.1, 138.3, 136.2, 135.8, 135.0, 130.8, 129.4, 128.8, 123.8, 123.2, 122.3, 121.4, 118.2, 113.3, 109.4, 108.2, 103.2, 99.6, 37.7, 34.5, 21.1, 14.1. LC-MS: m/z = 339 (M + H)+, positive mode; Anal. calcd for molecular formula C24H22N2; C, 85.17; H, 6.55; N, 8.28%; found: C, 85.31; H, 6.45; N, 8.21%.
5-Ethyl-2,4,10-trimethyl-1,5-dihydropyrrolo[3,2-b]carbazole (3n)
Brown colored semi-solid; yield: 67%, IR (KBr): 3408, 2974, 1660, 1553, 1452, 1280, 858, 821, 758, 632, 435 cm−1; hexane–EtOAc (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); Rf = 0.68. 1H NMR (400 MHz, TMS, CDCl3): δ 8.04 (s, 1H), 7.43–7.41 (m, 1H), 7.25–7.23 (m, 1H), 7.06 (s, 1H), 7.03 (d, 1H, J = 7.2 Hz), 6.88–6.86 (m, 1H), 4.68 (q, 2H, J = 7.08 Hz), 3.21 (s, 3H), 2.84 (s, 3H), 2.53 (s, 3H), 1.43 (t, 3H, J = 7.12 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 138.2, 136.0, 133.5, 131.4, 129.8, 127.5, 123.8, 122.5, 120.4, 117.1, 114.5, 109.4, 103.3, 102.7, 39.4, 24.7, 20.7, 15.6, 13.8. LC-MS: m/z = 277 (M + H)+, positive mode; Anal. calcd for molecular formula C19H20N2; C, 82.57; H, 7.29; N, 10.14%; found: C, 82.68; H, 7.21; N, 10.21%.
3); Rf = 0.68. 1H NMR (400 MHz, TMS, CDCl3): δ 8.04 (s, 1H), 7.43–7.41 (m, 1H), 7.25–7.23 (m, 1H), 7.06 (s, 1H), 7.03 (d, 1H, J = 7.2 Hz), 6.88–6.86 (m, 1H), 4.68 (q, 2H, J = 7.08 Hz), 3.21 (s, 3H), 2.84 (s, 3H), 2.53 (s, 3H), 1.43 (t, 3H, J = 7.12 Hz). 13C NMR (100 MHz, TMS, CDCl3): δ 138.2, 136.0, 133.5, 131.4, 129.8, 127.5, 123.8, 122.5, 120.4, 117.1, 114.5, 109.4, 103.3, 102.7, 39.4, 24.7, 20.7, 15.6, 13.8. LC-MS: m/z = 277 (M + H)+, positive mode; Anal. calcd for molecular formula C19H20N2; C, 82.57; H, 7.29; N, 10.14%; found: C, 82.68; H, 7.21; N, 10.21%.
Acknowledgements
We gratefully acknowledge DST (project number: SR/S1/OC-70/2008) for financial support and for the single-crystal X-ray diffractometer facility in our school. M. V. thanks CSIR for a senior research fellowship. M. V. also thanks Nagaraju, Balawardhan Rao for help with crystal studies, and MSc project student Kumaraswamy.References
- (a) H.-J. Knölker, K. R. Reddy and A. W. Schmidt, Chem. Rev., 2012, 112, 3193–3328 CrossRef; (b) H.-J. Knölker and K. R. Reddy, in The Alkaloids, ed. G. A. Cordell, Academic Press, Amsterdam, 2008, 65, pp. 1–430 Search PubMed; (c) I. Bauer and H.-J. Knölker, Synthesis of Pyrrole and Carbazole Alkaloids, Topp. Curr. Chem., 2012, 309, 203–254 Search PubMed; (d) H.-J. Knölker, Curr. Org. Synth., 2004, 1, 309–331 CrossRef; (e) G. W. Gribble, in The Alkaloids, ed. A. Brossi, Academic Press, San Diego, CA, 1990, pp. 239–352 Search PubMed; (f) P. Molina, P. M. Fresneda and P. Almendros, Tetrahedron, 1993, 49, 1223–1236 CrossRef CAS; (g) G. H. Kirsch, Curr. Org. Chem., 2001, 5, 507–518 CrossRef CAS; (h) J. Bergman, T. Janosik and N. Wahlstrom, Adv. Heterocycl. Chem., 2001, 80, 1–71 CrossRef CAS; (i) F. Bellina and R. Rossi, Tetrahedron, 2006, 62, 7213–7256 CrossRef CAS; (j) C. Sánchez, C. Méndez and J. A. Salas, Nat. Prod. Rep., 2006, 23, 1007–1045 RSC; (k) H.-P. Shi, J.-X. Dai, L. Xu, L.-W. Shi, L. Fang, S.-M. Shuanga and C. Dong, Org. Biomol. Chem., 2012, 10, 3852–3858 RSC; (l) E. Kianmehr and M. Ghanbari, Eur. J. Org. Chem., 2012, 256–259 CrossRef CAS; (m) K. K. Gruner, T. Hopfmann, K Matsumoto, A. Jäger, T. Katsuki and H.-J. Knölker, Org. Biomol. Chem., 2011, 9, 2057–2061 RSC; (n) T. Tsuchimoto, H. Matsubayashi, M. Kaneko, Y. Nagase, T. Miyamura and E. Shirakawa, J. Am. Chem. Soc., 2008, 130, 15823–15835 CrossRef CAS; (o) T. Tsuchimoto, H. Matsubayashi, M. Kaneko, E. Shirakawa and Y. Kawakami, Angew. Chem., Int. Ed., 2005, 44, 1336–1340 CrossRef CAS; (p) Y. Wu, H. Guo, T. D. James and J. Zhao, J. Org. Chem., 2011, 76, 5685–5695 CrossRef CAS.
- (a) S. L. Rodriguez, G. E. Zaballos, R. E. Gonzalez, M. L. Testa, A. J. Sepulveda and R. A. Jones, Tetrahedron, 2000, 56, 4511–4514 CrossRef; (b) R. L. Hudkins, N. W. Johnson, T. S. Angeles, G. W. Gessner and J. P. Mallamo, J. Med. Chem., 2007, 50, 433–441 CrossRef CAS; (c) M. A. Fousteris, A. Papakyriakou, A. Koutsourea, M. Manioudaki, E. Lampropoulou, E. Papadimitriou, G. A. Spyroulias and S. S. Nikolaropoulos, J. Med. Chem., 2008, 51, 1048–1052 CrossRef CAS; (d) M. Chakrabarty and S. Khasnobis, J. Indian Chem. Soc., 1997, 74, 917–925 CAS; (e) U. Pindur and T. Lemster, Recent Res. Dev. Org. Bioorg. Chem., 1997, 1, 33–54 CAS; (f) W. Fröhner, M. P. Krahl, K. R. Reddy and H.-J. Knölker, Heterocycles, 2004, 63, 2393–2407 CrossRef; (g) S. Agarwal, S. Cämmerer, S. Filali, W. Fröhner, J. Knoll, M. P. Krahl, K. R. Reddy and H.-J. Knölker, Curr. Org. Chem., 2005, 9, 1601–1614 CrossRef CAS; (h) U. Pindur, Y.-S. Kim and F. Mehrabani, Curr. Med. Chem., 1999, 6, 29–69 CAS; (i) M. Prudhomme, Eur. J. Med. Chem., 2003, 38, 123–140 CrossRef CAS; (j) M. Prudhomme, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 509–521 CrossRef CAS; (k) P. Appukkuttan, E. Van der Eycken and W. Dehaen, Synlett, 2005, 127–133 CAS; (l) L. Ackermann and A. Althammer, Angew. Chem., Int. Ed., 2007, 46, 1627–1629 CrossRef CAS.
- (a) R. Akué-Gédu, E. Rossignol, S. Azzaro, S. Knapp, P. Filippakopoulos, A. N. Bullock, J. Bain, P. Cohen, M. Prudhomme, F. Anizon and P. Moreau, J. Med. Chem., 2009, 52, 6369–6381 CrossRef; (b) B. Hugon, F. Anizon, C. Bailly, R. M. Golsteyn, A. Pierre, S. Leonce, J. Hickman, B. Pfeiffer and M. Prudhomme, Bioorg. Med. Chem., 2007, 15, 5965–5980 CrossRef CAS; (c) S. Deslandes, S. Chassaing and E. Delfourne, Mar. Drugs, 2009, 7, 754–786 CrossRef CAS; (d) M. Viji and R. Nagarajan, Tetrahedron, 2012, 68, 2453–2458 CrossRef CAS.
- (a) K. Warabi, S. Matsunaga, R. Soest and N. Fusetani, J. Org. Chem., 2003, 68, 2765–2770 CrossRef CAS; (b) A. Fürstner, M. M. Domostoj and B. Scheiper, J. Am. Chem. Soc., 2005, 127, 11620–11621 CrossRef; (c) A. Fürstner, M. M. Domostoj and B. Scheiper, J. Am. Chem. Soc., 2006, 128, 8087–8094 CrossRef; (d) P. Buchgraber, M. M. Domostoj, B. Scheiper, C. Wirtz, R. Mynott, J. Rust and A. Fürstner, Tetrahedron, 2009, 65, 6519–6534 CrossRef CAS; (e) K. Okano, H. Fujiwara, T. Noji, T. Fukuyama and H. Tokuyama, Angew. Chem., Int. Ed., 2010, 49, 5925–5929 CAS; (f) H. Tokuyama, K. Okano, H. Fujiwara, T. Noji and T. Fukuyama, Chem.–Asian J., 2011, 6, 560–572 CrossRef CAS.
- (a) K. B. Hong, C. W. Lee and E. K. Yuma, Tetrahedron Lett., 2004, 45, 693–697 CrossRef CAS; (b) G. Raffa, M. Rusch, G. Balme and N. Monteiro, Org. Lett., 2009, 11, 5254–5257 CrossRef CAS; (c) Y.-T. Su, Y.-L. Wang and G.-W. Wang, Chem. Commun., 2012, 48, 8132 RSC; (d) J. Li, Y. Yu, M.-S. Tu, B. Jiang, S.-L. Wang and S.-J. Tu, Org. Biomol. Chem., 2012, 10, 5361–5365 RSC; (e) E. Henon and J. Sapi, Org. Biomol. Chem., 2010, 8, 4625–4636 RSC; (f) M. Livecchi, G. Calvet and F. Schmidt, J. Org. Chem., 2012, 77, 5006–5016 CrossRef CAS; (g) H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 7318–7322 CrossRef CAS.
- (a) M. C. Willis, G. N. Brace and I. P. Holmes, Angew. Chem., Int. Ed., 2005, 44, 403 CrossRef CAS; (b) L. Ackermann, Org. Lett., 2005, 7, 439 CrossRef CAS; (c) H.-C. Zhang, H. Ye, A. F. Moretto, K. K. Brumfield and B. E. Maryanoff, Org. Lett., 2000, 2, 89 CrossRef CAS; (d) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1470 CrossRef CAS; (e) P. Müller, C. Baud and Y. Jacquier, Tetrahedron, 1996, 52, 1543–1548 CrossRef; (f) For general reviews see: Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed; (g) L. S. Hegedus, Transition Metals In the Synthesis of Complex Organic Molecules, University Science Book, Mill Valley, CA, 1994 Search PubMed; (h) J. J. Li and G. W. Gribble, Palladium in Heterocyclic Chemistry: A Guide for the Synthetic Chemist, Pergamon, Oxford, 2000 Search PubMed; (i) N. Miyaura, Cross-Coupling Reactions: A Practical Guide, Topics in Current Chemistry, 2002, vol. 219 Search PubMed; (j) I. J. S. Fairlamb, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2004, 100, 113–148 RSC; (k) B. B. Snider, Chem. Rev., 1996, 96, 339–363 CrossRef CAS; (l) T. Hirao, Chem. Rev., 1997, 97, 2707–2724 CrossRef CAS.
- (a) M. P. Kumar and R.-S. Liu, J. Org. Chem., 2006, 71, 4951–4955 CrossRef CAS; (b) M. Tokunaga, M. Ota, M. A. Hagac and Y. Wakatsukib, Tetrahedron Lett., 2001, 42, 3865–3868 CrossRef CAS; (c) S. Murarka and A. Studer, Org. Lett., 2011, 13, 2746–2749 CrossRef CAS; (d) J. R. Calvin, M. O. Frederick, D. L. T. Laird, J. R. Remacle and S. A. May, Org. Lett., 2012, 14, 1038–1041 CrossRef CAS; (e) G.-Q. Liu and Y.-M. Li, Tetrahedron Lett., 2011, 52, 7168–7170 CrossRef CAS; (f) M.-C. Tseng and Y.-H. Chu, Tetrahedron, 2008, 64, 9515–9520 CrossRef CAS; (g) M.-S. Mudadu, A.-S. Singh and R. P. Thummel, J. Org. Chem., 2008, 73, 6513–6520 CrossRef CAS; (h) H. Y. Lee, G. S. Chen, C.-S. Chen and J.-W. Chern, J. Heterocycl. Chem., 2010, 47, 454–458 CAS; (i) L. Zhou and M. P. Doyle, Org. Lett., 2010, 12, 796–799 CrossRef CAS; (j) K. Alex, A. Tillack, N. Schwarz and M. Beller, Tetrahedron Lett., 2008, 49, 4607–4609 CrossRef CAS; (k) V. Srinivas, K. V. Sajna and K. C. Kumara Swamy, Tetrahedron Lett., 2011, 52, 5323–5326 CrossRef CAS; (l) A. Rostami, A. Colin, X. Y. Li, M. G. Chudzinski, A. J. Lough and M. S. Taylor, J. Org. Chem., 2010, 75, 3983–3992 CrossRef CAS; (m) J. M. O'Brien and J. S. Kingsbury, J. Org. Chem., 2011, 76, 1662–1672 CrossRef CAS; (n) S. Yamazaki, M. Takebayashi and K. Miyazaki, J. Org. Chem., 2010, 75, 1188–1196 CrossRef CAS.
- (a) J.-S. Stover, J. Shi, W. Jin, P.-K. Vogt and D.-L. Boger, J. Am. Chem. Soc., 2009, 131, 3342–3348 CrossRef CAS; (b) H. Chabane, C. Lamazzi, V. Thiéry, G. Guillaumet and T. Besson, Tetrahedron Lett., 2002, 43, 2483–2486 CrossRef CAS; (c) L.-A. Estrada and D.-C. Neckers, Org. Lett., 2011, 13, 3304–3307 CrossRef CAS; (d) J. Panteleev, R. Y. Huang, E. K. J. Lui and M. Lautens, Org. Lett., 2011, 13, 5314–5317 CrossRef CAS; (e) C. G. Nasveschuk and T. Rovis, Angew. Chem., Int. Ed., 2005, 44, 3264–3267 CrossRef CAS; (f) A. Godt, J. Org. Chem., 1997, 62, 7471–7474 CrossRef CAS.
- The CCDC deposition number for compound 3f is 893524; molecular formula: C17H17N2Cl2. Chemical formula weight is 317.21; orthorhombic; unit cell parameters: a = 6.0170(5) Å, b = 8.9910(8) Å, c = 27.632(3) Å, space group P212121. The CCDC deposition number for compound 3k is 893523; molecular formula: C23H20N2. Chemical formula weight is 324.16; orthorhombic; unit cell parameters: a = 15.7399(15) Å, b = 8.8045(8) Å, c = 24.485(2) Å, space group Pbca.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 893523–893524. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21735j |
| This journal is © The Royal Society of Chemistry 2012 |