Fluorescent carbon dots obtained from chitosan gel†
Devasish
Chowdhury
*a,
Neelam
Gogoi
a and
Gitanjali
Majumdar
b
aPhysical Sciences Division, Polymer Unit, Institute of Advanced Study in Science and Technology, Paschim Boragaon, Garchuk, Guwahati, 781035, India. E-mail: devasishc@gmail.com; Fax: +91 361 2279909; Tel: +91 361 2912073
bDepartment of Chemistry, Assam Engineering College, Jalukbari, Guwahati, 781013, India. E-mail: gitanjalic@gmail.com; Fax: +91 361 2572215; Tel: +91 3612570550
First published on 18th October 2012
Abstract
In this communication we report the preparation of fluorescent carbon dots (CDs) from chitosan gel. We also studied the photoluminescent (PL) properties of CDs prepared in different pH conditions. While CDs prepared at pH 3 give an intense sharp blue fluorescence, those prepared at pH 1 or 5 give a broad emission. In addition, we also investigated CDs prepared from chitosan/Ag and chitosan/Au nanocomposites. It was observed that although the emission was broad and less well defined, there was an enhancement of PL emission for the CDs prepared from chitosan/Ag or chitosan/Au nanocomposites.
In recent years there has been a huge interest in carbon nanomaterials primarily because of their unique properties. Carbon nanomaterials discovered previously which have become a hot research topic are carbon nanotubes (SWNT)1–4 and graphene.5–8 A new class of carbon nanomaterials that have been recently discovered are carbon dots (CDs), which have attracted enormous attention due to their application in optoelectronic devices, biomedical imaging,9–11 biological labelling12 and drug delivery.13,14 These photoluminescent materials are chemically stable and environmentally and biologically compatible9,11,15 compared to semiconductor quantum dots like CdS, CdSe, ZnS etc., which have known toxicity and are potentially environmentally hazardous as they essentially contain heavy metals.
Most of the synthetic methods developed for the synthesis of CDs involve thermal decomposition, laser ablation, thermal oxidation or electro-oxidation of carbonaceous sources. To the best of our knowledge, the first report of the preparation of quantum sized carbon dots was reported by Sun et al., who produced bright colourful photoluminescent quantum sized carbon dots by laser ablation of a carbon target in the presence of water vapour, with argon as a carrier gas and using diamine terminated oligomeric poly(ethylene glycol) as a surface passivating agent.16 Mochalin et al.17 reported a route to produce hydrophobic blue fluorescent nanodiamonds by covalently linking octadecylamine to 5 nm nanodiamonds. Bourlinos et al.18 reported the synthesis of carbon dots by the thermal oxidation of a novel salt precursor made from the acid–base combination of tris(hydromethyl) aminomethane (Tris) and betaine hydrochloride into the corresponding ammonium carboxylate complex. Peng et al. prepared luminescent carbogenic dots (due to the presence of oxygen) using carbohydrates.19 Jaiswal et al. reported a one-step microwave mediated method for synthesizing carbon dots using PEG as a precursor and passivating agent.20 In a similar study, Wang et al.21 presented a one-step synthetic route for producing carbon dots using carbohydrates like glycerol, glycol, glucose, sucrose, etc., and a tiny amount of an inorganic ion. There are reports in the literature of producing carbon dots from activated carbon and candle soot22,23 Zhang et al. prepared green luminescent carbon dots by carbonization of sucrose. The non-luminous carbon nanoparticles were separated by dialysis. They also showed that after surface functionalization with PEG, the non-luminous nanoparticles became blue emitters.24
In this communication we report a simple method for the preparation of fluorescent carbon dots (CDs) from chitosan gel. It is the first reported method of obtaining CDs from chitosan gel. The advantage of using a gel is that it is stable for years without any degradation, so it will increase the shelf life of the source material for preparing CDs (i.e. chitosan). Moreover, the PL emission of chitosan gel is sharp, unlike that of chitosan solution where a broad peak is observed (details in the ESI†). We also studied the photoluminescent properties of the CDs in the presence of acetic acid (AA) and hydrochloric acid (of different pH). In addition we investigated CDs prepared from chitosan/Ag and chitosan/Au nanocomposites synthesized by in situ and ex situ methods, labelled as chitosan/Ag in situ (CH–Ag-I) or chitosan/Au in situ (CH–Au-I), and chitosan/Ag ex situ (CH–Ag-E) or chitosan/Au ex situ (CH–Au-E) nanocomposites, respectively (details of experimental procedure and nanoparticles (NPs) preparation is given in the ESI†).
The chitosan (CH) hydrogels were synthesized using a simple approach. 1% glacial acetic acid solution and glycerol were mixed in the ratio of 1 part acetic acid solution to 3 parts glycerol to form a solvent. 0.1 g chitosan was dissolved in 10 ml of the aforementioned solvent by stirring with a magnetic stirrer at room temperature for 1–2 h to form a clear pale yellow solution. The solution was neutralized by adding 5 N NaOH. Immediately a clear, slightly tacky gel was formed. The gel, which apparently results from the interaction of chitosan, glycerol and water, has a three dimensional structure, and no free water or glycerol is apparent.
To make the CDs, a small part of the CH hydrogel was taken and dissolved in 0.1 M acetic acid and heated in a microwave for 5 mins. Fig. 1(A) shows the schematic representation of the procedure used to obtain the carbon dots (CDs) from the chitosan gel. The representative scanning electron microscope image of the CDs is shown in Fig. 1(B). The particle size of the CDs prepared from the chitosan gel was also determined by dynamic light scattering (DLS) measurements. The DLS data showed that the particle sizes of CDs are in the range of 0.6 nm to 8.7 nm (Fig. 1(C)). Fig. 2 shows the photoluminescence (PL) spectra of CDs excited at different wavelengths. It was observed that with an increase in the excitation wavelength from 300 nm to 400 nm, the emission of the CDs gradually shifted to higher wavelengths accompanied with decreased fluorescence intensity. This red shift in the emission of CDs has also been reported previously.20 It was observed that excitation at 300, 310, 320, 330, 340, 350 and 400 nm resulted in emission at 334, 347, 358, 371, 384, 397 and 461 nm, respectively. As far as the PL mechanism of CDs is concerned, it is believed that both the quantum size effect and surface defects contribute to the PL. The inset of Fig. 2 shows a photograph of a CDs solution when viewed under a UV lamp. The zeta potential of the prepared CDs was found to be 27 mV, indicating the positive charge on the CDs. It is worth mentioning here that CDs are not formed by the microwave heating of glycerol and acetic solution and no fluorescence was observed, confirming that chitosan acts as a carbon source for the formation of CDs.
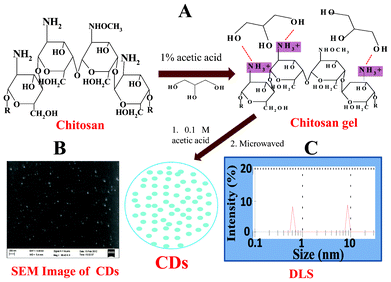 | ||
| Fig. 1 (A) Schematic representation of the procedure used to obtain carbon dots (CDs) from chitosan gel, (B) representative scanning electron microscope image of the CDs, and (C) particle size distribution of the CDs obtained from DLS. | ||
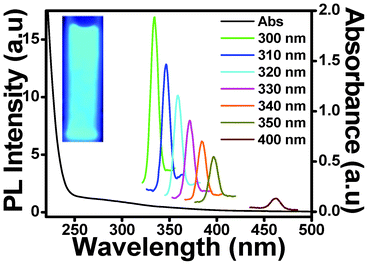 | ||
| Fig. 2 Photoluminescence emission and absorption spectra of carbon dots (CDs) obtained from chitosan gel using 0.1 M acetic acid (pH 3) at excitation wavelengths from 300 nm to 400 nm. Inset: photograph of the carbon dots after illumination under a UV lamp. | ||
We also tried to make CDs at different pH values. It was observed that the colour of the CD solution became darker at lower pH. The colour of the CD solution is dark yellow at pH 1 (CDs prepared in 0.1 M HCl), pale yellow at pH 3 (CDs prepared in 0.1 M AA), and off white at pH 5 (CDs prepared in 0.005 M AA). This change in colour of the CD solution suggests a change in the electronic transition of π–π* and n–π* in the carbon nanodomain at lower pH.25 The PL spectra of CDs obtained using 0.1 M hydrochloric acid (pH 1) after excitation at different wavelengths from 310 nm to 400 nm is shown in Fig. 3(A). The emission spectra show that the spectrum profile is a little different when compared with that of the CDs produced using 0.1 M acetic acid, shown in Fig. 2 (pH 3). There is a sharp emission peak at a similar wavelength followed by a broad peak, with red shift and decrease in intensity observed on increasing the excitation wavelength from 310 to 400 nm. It was observed that excitation at 320 nm gave a sharp emission at 357 nm followed by broad peak at 396 nm. CDs prepared using 0.1 M acetic acid (pH 3) gave emission at 358 nm with same excitation wavelength. Fig. 3(B) shows the PL emission spectra of CDs prepared in presence of 0.005 M AA (pH 5) excited at different wavelengths from 310 nm to 400 nm. In this case too the PL emission spectra are broad and are more shifted with increased excitation wavelength. For example, excitation at 320 nm gave a sharp emission at 359 nm and a broad emission at 402 nm. It was observed that the CDs prepared at pH 3 showed a smaller particle size distribution. For the CDs prepared at pH 1 and pH 5, the particle size distributions as obtained from DLS showed a bigger distribution of particles, and hence the PL emission was also broad and two emission peaks were obtained. In fact it was also found that it was not possible to measure the zeta potential of the CDs obtained at pH 1, perhaps because of the polydisperse nature of the particle size. Hence the pH value affects the fluorescence properties of the CDs, and the emission can be tuned by changing the pH.
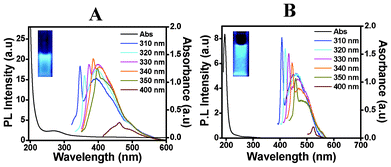 | ||
| Fig. 3 Photoluminescence emission and absorption spectra, obtained with excitation wavelengths from 310 nm to 400 nm, of carbon dots (CDs) obtained from chitosan gel using (A) 0.1 M hydrochloric acid (pH 1) and (B) 5 × 10−3 M acetic acid (pH 5). | ||
It is now well known that the interaction of fluorophores like CDs with metal NPs results in increased photostability, fluorescence enhancement and decreased lifetime. This can be attributed to increased rates of system radioactive decay. The fluorescence enhancement observed in this system can be derived from surface plasmon resonance. The absorption/emission spectra of the CDs show excellent overlap with the silver scattering spectrum, enabling enhanced fluorescence. So we then tried to prepare CDs from ex situ and in situ chitosan-Ag nanocomposites and chitosan-Au nanocomposites. In CH–Ag-I, Ag NPs are incorporated while making the chitosan gel, and as a consequence the Ag NPs are electrostatically adsorbed in the 3-dimensional gel structure, and are thus quite stable. In such a structure Ag NPs are strongly bound to the gel structure. On the other hand, the CH–Ag-E nanocomposite is formed by placing the chitosan gel in an Ag NPs solution, resulting in electrostatic adsorption of the NPs onto the already formed gel structure. So, the electrostatically adsorbed Ag NPs are loosely bound on the surface.26
Fig. 4(A) and (B) show the photoluminescence emission spectra of CDs formed from chitosan gel, CDs obtained from CH–Ag-I and CH–Ag-E nanocomposites, termed as Ag-I/CDs and Ag-E/CDs, respectively, and CDs obtained from CH–Au-I and CH–Au-E nanocomposites, termed as Au-I/CDs and Au-E/CDs, respectively. It is evident from the spectra that there is an enhancement of emission intensity in the case of Ag-I/CDs and Au-I/CDs when compared with the CDs obtained from chitosan gel. However the emission peaks are broad and less well defined. The reason for the broad peak is that CH–Ag-I, CH–Ag-E, CH–Au-I and CH–Au-E give more polydisperse CDs (bigger size) than those prepared from CH gel. The degree of metal–fluorophore interaction strongly depends on the distance between the fluorophore and the metal surface.27,28 We think that the enhancement of photoluminescence intensity shown by Ag-I/CDs and Au-I/CDs is due to the fact that the distance between the CDs and Ag is shorter in Ag-I/CDs than in Ag-E/CDs. As Ag and Au are loosely bound in CH–Ag-E and CH–Au-E, the interaction between the CDs and Ag or Au is weaker. More experiments are required in this regard. We are working on finding the answers to the following questions: (1) what is the interaction with different sizes of metal nanoparticles, and (2) whether the interaction of Ag NPs with chitosan, which is of course different in CH–Ag-I and CH–Ag-E, has any role when preparing CDs from the CH–Ag-I and CH–Au-E nanocomposites (in CH–Ag-I the electrostatically adsorbed Ag NPs form a 3-dimensional gel structure which is thus quite stable. On the other hand, in CH–Ag-E the electrostatically adsorbed Ag NPs are loosely bound). Moreover, the particle size distributions of the Ag-I/CDs, Ag-E/CDs, Au-I/CDs and Au-E/CDs reveal that the Ag-E/CDs or Au-E/CDs have bigger particle sizes than Ag-I/CDs or Ag-I/CDs (details given in the ESI†). Photoluminescence emission and absorption spectra obtained with progressively longer excitation wavelengths from 300 nm to 400 nm of the Ag-I/CDs, Ag-E/CDs, Au-I/CDs and Au-E/CDs are shown in the ESI.† It is also interesting to note that the net surface charge on the Ag-I/CDs and Ag-E/CDs obtained from the zeta potential was determined to be positive, like the CDs obtained from chitosan gel (details provided in the ESI†).
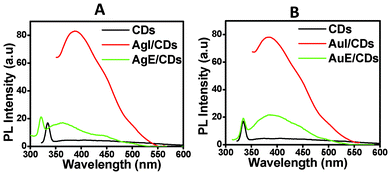 | ||
| Fig. 4 Photoluminescence emission spectra obtained from CDs excited at 300 nm, obtained from (A) Ag-I/CDs and Ag-E/CDs, and (B) Au-I/CDs and Au-E/CDs. | ||
Conclusions
In this communication we successfully prepared fluorescent carbon dots (CDs) from chitosan gel. The particle sizes of the CDs were in the range of 0.6 nm to 8.7 nm. PL studies on the CDs prepared at different pH conditions showed that, while the CDs prepared at pH 3 gave an intense sharp blue fluorescence, those prepared at pH 1 and pH 5 show broad emission. In addition, CDs prepared from CH–Ag-I, CH–Au-I, CH–Ag-E, and CH–Au-E showed enhancement of emission intensity in the case of Ag-I/CDs and Au-I/CDs when compared with Ag-E/CDs, Au-E/CDs, and CDs obtained from chitosan gel. This enhancement is attributed to the stronger interaction of Ag or Au in the Ag-I/CDs and Au-I/CDs. This work demonstrates a simple strategy for preparing CDs from chitosan gel, which possess good PL properties, low cytotoxicity and biocompatibility, and can find applications in biological labelling, biological imaging and in biosensors.Acknowledgements
The authors would like to thank the Council of Scientific and Industrial Research (CSIR), New Delhi for the project No. 01(2488)/11/EMR-II. NG thanks CSIR, New Delhi for a fellowship. The authors would also like to thank the reviewers for their critical comments, which helped to improve the manuscript.References
- P. Avouris, Acc. Chem. Res., 2002, 35, 1026–1034 CrossRef CAS.
- Ali Javey, Jing Guo, Qian Wang, Mark Lundstrom and Hongjie Dai, Nature, 2003, 424, 654 CrossRef CAS.
- Jian Chen, Mark A. Hamon, Hui Hu, Yongsheng Chen, Apparao M. Rao, Peter C. Eklund and Robert C. Haddon, Science, 1998, 282, 95 CrossRef CAS.
- H. Baughman Ray, Changxing Cui, Anvar A. Zakhidov, Zafar Iqbal, Joseph N. Barisci, Geoff M. Spinks, Gordon G. Wallace, Alberto Mazzoldi, Danilo De Rossi, Andrew G. Rinzler, Oliver Jaschinski, Siegmar Roth and Miklos Kertesz, Science, 1999, 284, 1340–1344 CrossRef.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- A. Kumar, A. L. M. Reddy, A. Mukherjee, M. Dubey, X. Zhan, N. Singh, L. Ci, W. Edward Billups, J. Nagurny, G. Mital and P. M. Ajayan, ACS Nano, 2011, 5, 4345–4349 CrossRef CAS.
- Rak-Hwan Kim, Myung-Ho Bae, Dae Gon Kim, Huanyu Cheng, Bong Hoon Kim, Dae-Hyeong Kim, Ming Li, Jian Wu, Frank Du, Hoon-Sik Kim, Stanley Kim, David Estrada, Suck Won Hong, Yonggang Huang, Eric Pop and John A. Rogers, Nano Lett., 2011, 11, 3881–3886 CrossRef CAS.
- Claire Berger, Zhimin Song, Xuebin Li, Xiaosong Wu, Nate Brown, Cécile Naud, Didier Mayou, Tianbo Li, Joanna Hass, Alexei N. Marchenkov, Edward H. Conrad, Phillip N. First and Walt A. de Heer, Science, 2006, 312, 1191–1196 CrossRef CAS.
- Li Cao, Xin Wang, Mohammed J. Meziani, Fushen Lu, Haifang Wang, Pengju G. Luo, Yi Lin, Barbara A. Harruff, L. Monica Veca, Davoy Murray, Su-Yuan Xie and Ya-Ping Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS.
- Sheng-Tao Yang, Xin Wang, Haifang Wang, Fushen Lu, Pengju G. Luo, Li Cao, Mohammed J. Meziani, Jia-Hui Liu, Yuanfang Liu, Min Chen, Yipu Huang and Ya-Ping Sun, J. Phys. Chem. C, 2009, 113, 18110 CAS.
- Sheng-Tao Yang, Li Cao, Pengju G. Luo, Fushen Lu, Xin Wang, Haifang Wang, Mohammed J. Meziani, Yuanfang Liu, Gang Qi and Ya-Ping Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS.
- Chi-Cheng Fu, Hsu-Yang Lee, Kowa Chen, Tsong-Shin Lim, Hsiao-Yun Wu, Po-Keng Lin, Pei-Kuen Wei, Pei-Hsi Tsao, Huan-Cheng Chang and Wunshain Fann, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 727–732 CrossRef CAS.
- T. D. Burchell, Carbon Materials for Advanced Technologies, Pergamon, New York, 1999 Search PubMed.
- O. A. Shenderova, V. V. Zhirnov and D. W. Brenner, Crit. Rev. Solid State Mater. Sci., 2002, 27, 227–356 CrossRef CAS.
- Sk Md Palashuddin, Amit Jaiswal, Anumita Paul, Siddhartha Sankar Ghosh and Arun Chattopadhyay, Nature Scientific Reports, 2012, 2, 383 Search PubMed.
- Ya-Ping Sun, Bing Zhou, Yi Lin, Wei Wang, K. A. Shiral Fernando, Pankaj Pathak, Mohammed Jaouad Meziani, Barbara A. Harruff, Xin Wang, Haifang Wang, Pengju G. Luo, Hua Yang, Muhammet Erkan Kose, Bailin Chen, L. Monica Veca and Su-Yuan Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS.
- V. N. Mochalin and Y. Gogotsi, J. Am. Chem. Soc., 2009, 131, 4594–4595 CrossRef CAS.
- A. B. Bourlinos, R. Zbořil, J. Petr, A. Bakandritsos, M. Krysmann and E. P. Giannelis, Chem. Mater., 2012, 24, 6–8 CrossRef CAS.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563–5565 CrossRef CAS.
- A. Jaiswal, S. S. Ghosh and A. Chattopadhyay, Chem. Commun., 2012, 48, 407 RSC.
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, J. Mater. Chem., 2011, 21, 2445 RSC.
- Z-A. Qiao, Y. Wang, Y. Gao, H. Li, T. Dai, Y. Liu and Q. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473 CrossRef CAS.
- J. C. Zhang, W. Q. Shen, D. Y. Pan, Z. W. Zhang, Y. G. Fang and M. H. Wu, New J. Chem., 2010, 34, 591–593 RSC.
- J-L. Chen and X-P Yan, Chem. Commun., 2011, 47, 3135–3137 RSC.
- N. Gogoi and D. Chowdhury, RSC Adv. Search PubMed , communicated.
- C. Li, Y. Zhu, X. Zhang, X. Yang and C. Li, RSC Adv., 2012, 2, 1765–1768 RSC.
- R. Pribik, K. Aslan, Y. Zhang and Chris D. Geddes, J. Phys. Chem. C, 2008, 112, 17969–17973 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: details of characterization techniques, particle size distribution, zeta potential measurements and fluorescence microscopy images. See DOI: 10.1039/c2ra21705h |
| This journal is © The Royal Society of Chemistry 2012 |
