Combining mussel-inspired chemistry and the Michael addition reaction to disperse carbon nanotubes†
Xiaoyong
Zhang
a,
Meiying
Liu
c,
Yaling
Zhang
a,
Bin
Yang
a,
Yan
Ji
a,
Lin
Feng
a,
Lei
Tao
*a,
Shuxi
Li
a and
Yen
Wei
*ab
aDepartment of Chemistry and Key Lab of Organic Optoelectronic & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing, 100084, P. R. China. E-mail: leitao@mail.tsinghua.edu.cn; weiyen@tsinghua.edu.cn
bKey Lab of Organic Optoelectronic & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing, 100084, P. R. China
cBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Organic Solids, Laboratory of New Materials, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China
First published on 18th October 2012
Abstract
An efficient and general strategy for the dispersion of multi-walled carbon nanotubes in water and organic media was developed for the first time via the combination of mussel-inspired chemistry and the Michael addition reaction.
Since their initial discovery in 1991, carbon nanotubes have gained intensive research interest due to their remarkable electrical, mechanical and chemical properties.1,2 They have shown promising applications in many areas such as optical devices, nanomedicine, energy conversion, evironmental treatment etc.3–14 For many of these applications, the efficient dispersion of carbon nanotubes is a prerequisite for their optimal performance. Over the past few decades, numerous studies have focused on the preparation of water and organic dispersible carbon nanotubes using various strategies.15–25 However, many of the previous methods suffered from limitations such as multistep procedures, structural damage, enhancement of toxicity, and harsh reaction conditions etc.26–36 Therefore, there is still a requirement to develop novel, facile and effecient methods for the dispersion of carbon nanotubes in aqueous and organic media.
Mussels are promiscuous fouling organisms and have shown strong attachment to virtually all types of inorganic and organic surfaces.37,38 Inspired by the strong adhesion properties of mussels to surfaces, Messersmith et al. demonstrated that dopamine (an adhesive in mussel foot proteins) could self-polymerize in an alkaline environment, thus forming films on a wide range of inorganic and organic materials, including noble metals, oxides, polymers, semiconductors, and ceramics.39 After their pioneering work, mussel-inspired chemistry has been rapidly applied in various fields, such as surface coating, medical adhesive and sealants as well as the surface functionalization of nanoparticles.40–48 Other than its strong and general ability for surface adhesion, another appealing feature of dopamine is its high reaction activity with thiol and amino groups. Thus, various molecules can be easily introduced into polydopamine (PDA) films through Michael addition.49,50 However, to the best of our knowledge, the combination of mussel-inspired chemistry and the Michael addition reaction for surface modifications of nanomaterials is not well studied.51
In the current work, the preparation of highly-dispersed multi-walled carbon nanotubes (CNTs) in aqueous and organic solutions by the combination of mussel inspired chemistry and the Michael addition reaction was reported for the first time. CNTs were first coated with PDA film via the self-polymerization of dopamine, and then 3-mercapto-1-propanesulfonic acid sodium salt (MPS) or normal dodecanethiol (NDM) were conjugated with PDA film via the Michael addition reaction (Scheme 1). The dispersion of CNT nanoparticles in water and dichloromethane was subsequently investigated. Given its simplicity and effectiveness, the method described here should become a general strategy for the surface modifications of nanomaterials for various applications.
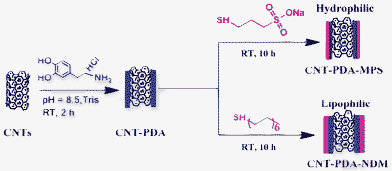 | ||
| Scheme 1 Schematic representation of the preparation of CNT-PDA-MPS and CNT-PDA-NDM via the combination of mussel-inspired chemistry and the Michael addition reaction. | ||
The dispersibilities of CNT-PDA-MPS and CNT-PDA-NDM in aqueous and organic solutions were investigated. As shown in Fig. 1A, pristine CNTs are quickly precipitated within 15 min (left bottle). In the sharp contrast, after surface modification with MPS, CNT nanoparticles (CNT-PDA-MPS) could be well-dispersed in water over several months (right bottle), suggesting that the hydrophilic molecule (MPS) was successfully conjugated on the surface of CNTs. However, after being surface modified with a lipophilic molecule (NDM), CNT nanoparticles (CNT-PDA-NDM) could be facily transferred from water into organic media after violent shaking for 5 min (Fig. 1B), further confirming the successful modification of CNTs with thiol-containing compounds.
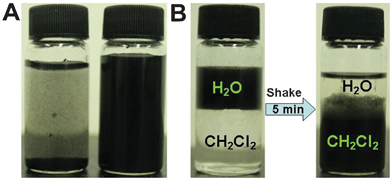 | ||
| Fig. 1 Dispersion of CNT nanoparticles in water and organic media. (A) The water dispersion of pristine CNTs (left bottle) and CNT-PDA-MPS (right bottle). (B) Transfer of CNT-PDA-NDM from water to dichloromethane. A clear water/oil interface was observed, in which CNT-PDA-NDM was dispersed in water (left bottle). Most of CNT-PDA-NDM was transferred to dichloromethane after shaking the bottle for 5 min (right bottle). | ||
Fig. 2 shows the representative TEM images of pristine and functionalized CNTs. It can be seen that the diameter of CNTs is about 30–50 nm, which is consistent with the information provided by the manufacturer. Compared with the pristine CNTs, the structure of the CNTs remained intact after surface modification with PDA and thiol-containing compounds (Fig. 2B–D), indicating that the properties of CNTs related to their structure were preserved. However, it is noteworthy that some PDA polymer films were present around the CNTs, and thus, the formation of low contrast areas was observed (As indicated by the arrows in Fig. 2C and 2D), providing direct evidence for the successful modification of CNTs.
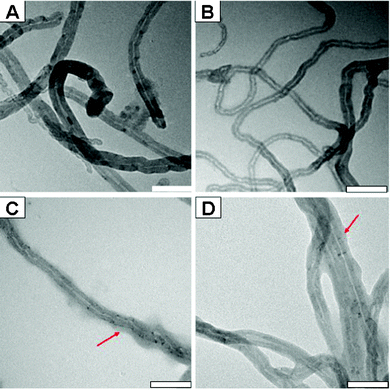 | ||
| Fig. 2 Representative TEM images of CNTs, CNT-PDA, CNT-PDA-MPS and CNT-PDA-NDM. Polymer films were clearly observed in the TEM images of C and D (red arrows); scale bar = 100 nm. | ||
Many other techniques, including Fourier transform infrared (FT-IR) spectrascopy, thermal gravimetric analysis (TGA), Raman spectrascopy and X-ray photoelectron spectroscopy (XPS), were further used to confirm the successful surface modifications of CNTs. As shown in Fig. 3A, an intensive peak located at 1377 cm−1, which can be attributed to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration mode, was observed in CNT-PDA-MPS, confirming the existence of MPS. Furthermore, two strong peaks located at 2908 and 2840 cm−1, corresponding to the stretching vibration bands of C–H, were also found in the sample of CNT-PDA-NDM, further confirming the successful surface modifications of CNTs with NDM.
O stretching vibration mode, was observed in CNT-PDA-MPS, confirming the existence of MPS. Furthermore, two strong peaks located at 2908 and 2840 cm−1, corresponding to the stretching vibration bands of C–H, were also found in the sample of CNT-PDA-NDM, further confirming the successful surface modifications of CNTs with NDM.
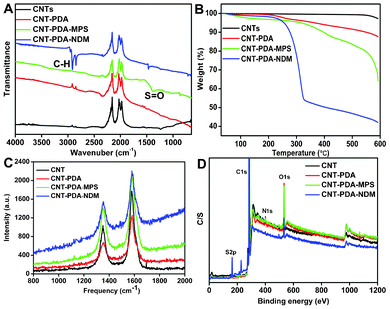 | ||
| Fig. 3 Characterization of CNTs, CNT-PDA, CNT-PDA-MPS and CNT-PDA-NDM. (A) FT-IR spectra, (B) TGA, (C) Raman spectra, and (D) XPS spectra. | ||
The TGA data give additional insight into the surface modifications of CNTs. It can be seen that weight loss of pristine CNTs is about 2.7%, suggesting the excellent thermal stability of CNTs. After surface modification with PDA, the weight loss of CNT-PDA was significantly increased to 12.5% (Fig. 3B). Therefore, the weight percentage of thePDA coating was about 9.8 wt% based on the TGA data. Upon further modification of CNT-PDA with MPS and NDM, the weight losses of CNT-PDA-MPS and CNT-PDA-NDM increased to 35.8 and 58.1%, respectively. These results further demonstrated the successful modifications of CNT-PDA with MPS and NDM via Michael addition. Fig. 3C showed the Raman spectra of CNT nanoparticles. Two characteristic peaks centered at about 1352 and 1582 cm−1, corresponding to the D and G bands of the CNTs, were observed in all CNT samples. However, compared with the pristine CNTs, the frequencies of the D and G bands of CNT-PDA-MPS and CNT-PDA-NDM were red-shifted about 2–3 cm−1. Additionally, the ratio values of ID/IG of CNT-PDA-MPS and CNT-PDA-NDM were slightly increased from 0.61 to 0.66 and 0.63, respectively (Table S1†). Raman results further indicated that the MPS and NDM molecules were successfully introduced onto the CNT surface.
Chemical compositions of the CNT nanoparticles were measured by XPS. The elements including carbon (C),oxygen (O), nitrogen (N) and sulfur (S) were confirmed by a survey XPS scan ranging from 0–1200 eV (Fig. 3D). As compared with pristine CNTs, N1s signals were observed on PDA-coated CNT nanoparticles (Fig. S2C†), evidencing the successful modification of CNT with PDA. Furthermore, S2p signals were observed for the samples of CNT-PDA-NDM and CNT-PDA-MPS, indicating that thiol-containing compounds could be further linked onto CNT-PDA. Additionally, the weight percentages of elements (C, N, O, S) were calculated based on the area of C1s, N1s, O1s and S2p. As listed in Table 1, only two elements (C and O) were detected on the surface of pristine CNTs, and their weight percentages are 97.13 (C) and 2.87 wt% (O), respectively. After surface coating with PDA, a new element, N (2.29%) was detected and the weight percentages of C and O were altered to 88.89 and 8.82 wt%, respectively.
| C | O | N | S | |
|---|---|---|---|---|
| CNTs | 97.13 | 2.87 | 0 | 0 |
| CNT-PDA | 88.89 | 8.82 | 2.29 | 0 |
| CNT-PDA-MPS | 89.77 | 8.35 | 1.25 | 0.63 |
| CNT-PDA-NDM | 89.67 | 5.04 | 1.08 | 4.2 |
Based on these results, the weight percentage of PDA coated on the surface of the CNTs is calculated to be 12.91 wt%, which is close to the results from the TGA data (9.8 wt%). Table 1 also demonstrated that thiol-containing compounds such as MPS and NDM could be further conjugated with PDA films through Michael addition. XPS analysis also confirmed the presence of S in CNT-PDA-MPS and CNT-PDA-NDM. However, it should also be noted that the weight percentage of S in CNT-PDA-NDM is significantly greater than in CNT-PDA-MPS. One possible reason is that NDM could be further incorporated into the lipophilic film conjugated to the surface of CNTs.
In summary, the surface modification of CNTs with MPS and NDM by the combination of mussel-inspired chemistry and Michael addition was reported for the first time. Both aqueous and organic dispersible CNTs were successfully obtained. In addition to MPS and NDM, many other thiol compounds with different functional groups such as –OH, –COOH and –NH2 might also be introduced onto CNTs by this facile method. Furthermore, given the strong adhesion between PDA and the CNT surface, this method could be further extended to the surface modification of other nanomaterials such as graphene, nano-diamond, iron oxide etc. Considering its simplicity and effectiveness, the procedure described here should be a facile and versatile method for the surface modification of nanomaterials and might find potential applications in various fields.
Acknowledgements
This research was supported by the National Science Foundation of China (no. 21104039, 21134004, 21201108), the National 973 Project (no. 2011CB935700), and the China Postdoctoral Science Foundation (2011M500280).References
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- Y. P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. De Heer, Science, 2002, 297, 787–792 CrossRef CAS.
- X. Qi, G. Poernomo, K. Wang, Y. Chen, M. B. Chan-Park, R. Xu and M. W. Chang, Nanoscale, 2011, 3, 1874–1880 RSC.
- L. Wei, N. Tezuka, T. Umeyama, H. Imahori and Y. Chen, Nanoscale, 2011, 3, 1845–1849 RSC.
- S. K. Vashist, D. Zheng, G. Pastorin, K. Al-Rubeaan, J. H. T. Luong and F. S. Sheu, Carbon, 2011, 49, 4077–4097 CrossRef CAS.
- A. Di Crescenzo, D. Velluto, J. A. Hubbell and A. Fontana, Nanoscale, 2011, 3, 925–928 RSC.
- N. W. S. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600 CrossRef CAS.
- R. J. Chen, S. Bangsaruntip, K. A. Drouvalakis, N. Wong Shi Kam, M. Shim, Y. Li, W. Kim, P. J. Utz and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 4984 CrossRef CAS.
- G. Wang, H. Wang, W. Li and J. Bai, RSC Adv., 2011, 1, 1585–1592 RSC.
- S. Chen, J. Zhu, H. Zhou and X. Wang, RSC Adv., 2011, 1, 484–489 RSC.
- Z. R. Tang, Y. Zhang and Y. J. Xu, RSC Adv., 2011, 1, 1772–1777 RSC.
- C. M. Hussain, C. Saridara and S. Mitra, RSC Adv., 2011, 1, 685–689 RSC.
- K. Qu, H. Xu, C. Zhao, J. Ren and X. Qu, RSC Adv., 2011, 1, 632–639 RSC.
- M. Islam, E. Rojas, D. Bergey, A. Johnson and A. Yodh, Nano Lett., 2003, 3, 269–273 CrossRef CAS.
- T. S. Jo, H. Han, L. Ma and P. K. Bhowmik, Polym. Chem., 2011, 2, 1953–1955 RSC.
- D. Tuncel, Nanoscale, 2011, 3, 3545–3554 RSC.
- S. Rana and J. W. Cho, Nanoscale, 2010, 2, 2550–2556 RSC.
- C. H. Liu and H. L. Zhang, Nanoscale, 2010, 2, 1901–1918 RSC.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, Nat. Mater., 2003, 2, 338–342 CrossRef CAS.
- C. Iamsamai, S. Hannongbua, U. Ruktanonchai, A. Soottitantawat and S. T. Dubas, Carbon, 2010, 48, 25–30 CrossRef CAS.
- Y. Yan, J. Cui, P. Pötschke and B. Voit, Carbon, 2010, 48, 2603–2612 CrossRef CAS.
- H. Nie, H. Wang, A. Cao, Z. Shi, S. T. Yang, Y. Yuan and Y. Liu, Nanoscale, 2011, 3, 970–973 RSC.
- X. Zhang, C. Fu, L. Feng, Y. Ji, L. Tao, Q. Huang, S. Li and Y. Wei, Polymer, 2012, 53, 3178–3184 CrossRef CAS.
- X. Zhang, S. Wang, C. Fu, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Polym. Chem., 2012, 3, 2716–2719 RSC.
- G. Xu, W. T. Wu, Y. Wang, W. Pang, Q. Zhu, P. Wang and Y. You, Polymer, 2006, 47, 5909–5918 CrossRef CAS.
- H. Kong, C. Gao and D. Yan, Macromolecules, 2004, 37, 4022–4030 CrossRef CAS.
- P. Wick, P. Manser, L. K. Limbach, U. Dettlaff-Weglikowska, F. Krumeich, S. Roth, W. J. Stark and A. Bruinink, Toxicol. Lett., 2007, 168, 121–131 CrossRef CAS.
- X. Zhang, Y. Zhu, J. Li, Z. Zhu, W. Li and Q. Huang, J. Nanopart. Res., 2011, 13, 1–12 CrossRef.
- X. Zhang, W. Hu, J. Li, L. Tao and Y. Wei, Toxicol. Res., 2012, 1, 62–68 RSC.
- Y. Zhu, W. Li, Q. Li, Y. Li, X. Zhang and Q. Huang, Carbon, 2009, 47, 1351–1358 CrossRef CAS.
- Y. Liang, X. Zhang, W. Luo, Z. Zhong, M. Lv, H. Feng, Y. Zhao and Q. Huang, Prep. Biochem. Biotechnol., 2011, 41, 243–251 CrossRef CAS.
- Y. Zhu, X. Zhang, J. Zhu, Q. Zhao, Y. Li, W. Li, C. Fan and Q. Huang, Int. J. Mol. Sci., 2012, 13, 12336–12348 CrossRef CAS.
- X. Zhang, J. Li, Y. Zhu, Y. Qi, Z. Zhu, W. Li and Q. Huang, Nucl. Sci. Tech., 2011, 22, 99–104 CAS.
- X. Zhang, J. Yin, C. Kang, J. Li, Y. Zhu, W. Li, Q. Huang and Z. Zhu, Toxicol. Lett., 2010, 198, 237–243 CrossRef CAS.
- X. Zhang, J. Yin, C. Peng, W. Hu, Z. Zhu, W. Li, C. Fan and Q. Huang, Carbon, 2011, 49, 986–995 CrossRef CAS.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS.
- J. Liu, Q. Ye, B. Yu, X. Wang and F. Zhou, Chem. Commun., 2012, 48, 398–400 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC.
- S. H. Ku and C. B. Park, Biomaterials, 2010, 31, 9431–9437 CrossRef CAS.
- H. Hu, B. Yu, Q. Ye, Y. Gu and F. Zhou, Carbon, 2010, 48, 2347–2353 CrossRef CAS.
- I. You, S. M. Kang, S. Lee, Y. O. Cho, J. B. Kim, S. B. Lee, Y. S. Nam and H. Lee, Angew. Chem., 2012, 124, 6230–6234 CrossRef.
- S. H. Yang, S. M. Kang, K. B. Lee, T. D. Chung, H. Lee and I. S. Choi, J. Am. Chem. Soc., 2011, 133, 2795–2797 CrossRef CAS.
- A. Postma, Y. Yan, Y. Wang, A. N. Zelikin, E. Tjipto and F. Caruso, Chem. Mater., 2009, 21, 3042–3044 CrossRef CAS.
- C. J. Ochs, T. Hong, G. K. Such, J. Cui, A. Postma and F. Caruso, Chem. Mater., 2011, 23, 3141–3143 CrossRef CAS.
- B. Yu, D. A. Wang, Q. Ye, F. Zhou and W. Liu, Chem. Commun., 2009, 45, 6789–6791 RSC.
- X. Zhang, S. Wang, L. Xu, Y. Ji, L. Feng, L. Tao, S. Li and Y. Wei, Nanoscale, 2012, 4, 5581–5584 RSC.
- H. Lee, J. Rho and P. B. Messersmith, Adv. Mater., 2009, 21, 431–434 CrossRef CAS.
- H. O. Ham, Z. Liu, K. Lau, H. Lee and P. B. Messersmith, Angew. Chem., 2011, 123, 758–762 CrossRef.
- P. Yan, J. Wang, L. Wang, B. Liu, Z. Lei and S. Yang, Appl. Surf. Sci., 2011, 257, 4849–4855 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: XPS spectra of C1s, N1s, O1s, S2p and Elemental content (%) of CNT nanoparticles based on XPS analysis. See DOI: 10.1039/c2ra22011c |
| This journal is © The Royal Society of Chemistry 2012 |
