Graphene oxide/titania hybrid films with dual-UV-responsive surfaces of tunable wettability†
Pengzhan
Sun
a,
Miao
Zhu
a,
Renzhi
Ma
b,
Kunlin
Wang
a,
Jinquan
Wei
a,
Dehai
Wu
a,
Takayoshi
Sasaki
b and
Hongwei
Zhu
*ac
aDepartment of Mechanical Engineering, Key Laboratory for Advanced Manufacturing by Materials Processing Technology, Tsinghua University, Beijing 100084, China. Fax: +86 10 62773637; Tel: +86 10 62781065E-mail: hongweizhu@tsinghua.edu.cn
bInternational Center for Materials Nanoarchitectonics, National Institute for Materials Science Tsukuba, Ibaraki 305–0044, Japan
cCenter for Nano and Micro Mechanics (CNMM), Tsinghua University, Beijing 100084, China
First published on 3rd September 2012
Abstract
Ultrathin hybrid films of graphene oxide (GO) and monolayer titania (TO) were assembled by layer-by-layer and drop-casting methods. The photo-induced wettability modulation of the hybrid films with different configurations was systematically studied. Due to the photocatalytic reduction of GO by TO, GO sheets in the hybrid films exhibited a tendency to undergo photo-induced conversion from hydrophilic to hydrophobic upon UV irradiation. On the contrary, TO nanosheets showed the reverse trend. Both surfaces of the hybrid film showed opposite yet tunable hydrophilicity under UV irradiation, demonstrating the potential for future application in liquid transport engineering.
1. Introduction
It is of great importance to control the wettability of solid surfaces in various fields such as coating and printing as well as adhesion.1,2 Typically, the wettability can be varied by chemical modification of the surfaces3 and application of specific external factors such as temperature gradient,4 electric fields,5–7 light irradiation8,9 and so on. Among them, light irradiation is a potent method for controlling the wettability of materials owing to its easy accessibility, high conversion rate and destruction-free property.Much attention has been attracted to this area in recent years since the discovery of the photo-induced super hydrophilic properties of TiO2 films, which have been applied in various kinds of field such as for self-cleaning and anti-fogging glass, side mirrors, automobile tiles, household glazing as well as buildings.10–14 TiO2 films show amphiphilic properties under different conditions and a hydrophilic–hydrophobic conversion can be achieved reversibly by alternating UV irradiation and dark storage. At the same time, the mechanism of photo-induced hydrophilic conversion has been investigated extensively. The successful synthesis of monolayer titania (TO) nanosheets by chemical exfoliation of layered titanates helps to better understand the photo-induced hydrophilic conversion of the surfaces, which lies in the fact that due to the structure of the TO nanosheets it can be considered that the entire surface atoms are arranged two-dimensionally.15,16 The TO nanosheets could be assembled into multilayer films via the layer by layer (LBL) electrostatic adsorption method and the thickness of the lamellar multilayer films could be precisely controlled.17 The as-prepared multilayer films exhibited efficient UV absorption properties18 and excellent dielectric nature with dielectric constants of 90–140 in the 103–107 Hz range.19,20 Moreover, the LBL multilayer films exhibited excellent photoinduced hydrophilic conversion properties and the results from synchrotron radiation in-plane X-ray diffraction demonstrated that slight but significant structural changes occurred during the photo-induced hydrophilic conversion.21,22
Graphene, another two-dimensional monolayer material composed of sp2 hybridized carbon bonds in a honeycomb-like network, attracts much attention because of its unique and outstanding properties.23 The fabrication of graphene oxide (GO) via oxidation and exfoliation of graphite in aqueous solution is demonstrated to be a promising method because it is cost-effective and easy to scale up.24–26 There have been several methodologies for the reduction of GO, such as chemical reduction,27–29 thermal reduction30,31 as well as light reduction.32,33 The light reduction of GO with a photocatalyst like TiO2 is a promising method due to its environment friendliness and mild conditions. Moreover, graphene-based composites can be directly fabricated during the reduction procedure.32–34
GO sheet can be regarded as graphene decorated with oxygen functional groups on both sides of the sheet as well as around the edges.35 These oxygen containing functional groups make GO super hydrophilic. When GO sheets are reduced to rGO, the amount of oxygen functional groups will decrease and a greater extent of the π network will be restored within the graphene structure and thus it will result in hydrophobic surfaces. As monolayer TO nanosheets change from hydrophobic to hydrophilic upon UV irradiation, it is of great importance to investigate the hybridization of GO and TO nanosheets, which may result in the reduction of GO to rGO in the presence of TO sheets as well as the dual tunable hydrophilicity of the ultrathin hybrid films.
Herein, we wish to report the fabrication of ultrathin hybrid films of GO and TO (Ti0.87O2) monolayers by using the LBL as well as drop-casting (DC) methods. The ultraviolet light promoted the decomposition of the interlayer PDDA, the in situ reduction of GO and the surface modification of TO, resulting in the interesting dual tunable wettability of the hybrid films (Fig. 1a). The mechanism and potential applications of the hybrid films in photo-induced hydrophilic/hydrophobic conversion are discussed.
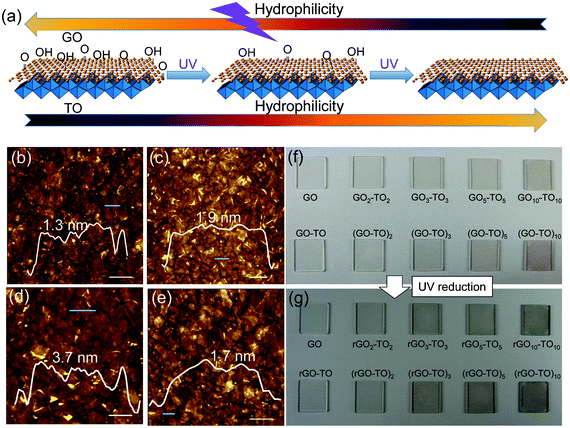 | ||
| Fig. 1 Dual tunable wettability of the TO/GO hybrid film. (a) Schematic diagram of the dual tunable hydrophilicity of the hybrid film. AFM images of (b) GO nanosheets, (c) TO nanosheets, (d) GO–TO film and (e) GO/TO hybrid film formed by LBL. The inset images show the height distributions of the nanosheets marked by green lines. Scale bars: 0.5 μm. (f) Photograph of as prepared samples of GOn–TOn and (GO–TO)n. The samples in the first row are GO, GO2–TO2, GO3–TO3, GO5–TO5, GO10–TO10. Samples of the second row are GO–TO, (GO–TO)2, (GO–TO)3, (GO–TO)5, (GO–TO)10. (g) Corresponding photographs of samples shown in (f) after UV irradiation for 48 h. | ||
2. Experimental
TO nanosheets were fabricated by exfoliating protonic titanate crystals according to our previous work.15,16 GO sheets were prepared by a modified Hummers' method from worm-like exfoliated graphite36 (see Experimental Section for details). The fabrication process of the hybrid samples formed by the LBL method is illustrated in Fig. S1a†: The surface-cleaned substrates (quartz glass or Si wafer) were first immersed in a PDDA solution (20 g L−1, pH = 9) for 20 min to introduce positive charges onto the substrates, and then thoroughly washed with deionized water. PDDA-treated substrates were immersed in GO (or TO, the corresponding photographs were shown in Fig. S2†) colloidal suspension for another 20 min (pH = 9) and then the substrates were rinsed with water. The LBL procedure was repeated to obtain GOn–TOn or TOn–GOn multilayer lamellar films of different configurations with the desired number of layers. The as-prepared films were dried under nitrogen flow. Finally, the samples were exposed to UV light for 48 h, during which time the PDDA layers were completely removed (decomposed) and GO was reduced to rGO (see XRD studies in the Experimental Section).The fabrication of the hybrid samples by the DC method is illustrated in Fig. S1b†: to obtain GOn–TOn or TOn–GOn lamellar films, the surface-cleaned substrates were drop-cast with ∼1 mL of 0.2 mg mL−1 GO colloidal suspension (or 0.16 mg mL−1 TO suspension) and dried in air at 80 °C. Then the GO (TO)-coated dry substrates were drop-cast with ∼1 mL of 0.16 mg mL−1 TO colloidal suspension (or 0.2 mg mL−1 GO suspension) and dried under the above-mentioned conditions. To obtain GO/TO hybrid films, the surface-cleaned substrates were drop-cast with ∼1 mL of a mixture of GO (0.2 mg mL−1) and TO (0.16 mg mL−1) colloidal suspensions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) and then the samples were dried in air at 80 °C.
1 in volume) and then the samples were dried in air at 80 °C.
3. Results and discussion
An atomic force microscopy (AFM) image of GO sheets deposited on Si wafer by LBL method is displayed in Fig. 1b. GO sheets adhered tightly on the Si wafer and the lateral size ranged from several tens of nanometers to several micrometers. Most of the GO sheets possessed a height of less than 2 nm and overlaps of several layers were also found. A typical AFM image of TO deposited on Si wafer by the LBL method is shown in Fig. 1c, revealing the self-assembly of TO nanosheets with more uniform coverage compared with previously reported result.34 Most of the monolayer nanosheets were distributed uniformly on the substrate, but there were also some patches showing overlapping as well as gaps between nanosheets. Nearly all of them had a lateral size of several hundred nanometers as well as a height of less than 2 nm for each sheet. An AFM image of the GO–TO lamellar films fabricated by the LBL method is displayed in Fig. 1d. It could be clearly observed that two layers of nanosheets packed together on the substrate and the film possessed a height of ∼4 nm, indicating that the GO and TO nanosheets maintain a fine laminate structure and uniformly assemble together. An AFM image of the GO/TO hybrid films fabricated by the LBL method from the GO (0.1 mg mL−1) and TO (0.08 mg mL−1) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) colloidal suspension is shown in Fig. 1e. GO and TO nanosheets were sporadically dispersed. Corresponding TEM images are shown in Fig. S3†. The interlayer spacing changes of the hybrid samples are revealed by X-ray diffraction (XRD) (see Fig. S4† and the Experimental Section for details).
1 in volume) colloidal suspension is shown in Fig. 1e. GO and TO nanosheets were sporadically dispersed. Corresponding TEM images are shown in Fig. S3†. The interlayer spacing changes of the hybrid samples are revealed by X-ray diffraction (XRD) (see Fig. S4† and the Experimental Section for details).
During the LBL process, the brown color of the sample became darker. For GOn–TOn and (GO–TO)n samples with the same n value, there was little difference in color (Fig. 1f), which should be attributed to the same number of TO and GO layers despite the different stacking sequence. After UV irradiation for 48 h, all the samples' color changed from brown to black (Fig. 1g), which revealed the effective reduction of GO to rGO in the presence of TO nanosheets.
We also collected the UV-vis absorption spectra of the samples fabricated by drop-casting and LBL methods as well as liquid phase hybrid samples. The GO/TO hybrid suspension was prepared by simply mixing two colloidal suspensions. Fig. S5,† of the TO colloidal suspension, shows a steep onset at around 324 nm, corresponding to the band gap of TO (∼3.84 eV). While no obvious absorption edge for GO suspension was observed, the spectrum only displayed an absorption peak located at ∼230 nm, which may be dominated by the π–π* transition,37 and a shoulder located at ∼300 nm, which may be attributed to the n–π transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.38,39 When mixing the two kinds of colloidal suspension, no obvious aggregates were observed, even after UV irradiation for different lengths of time (Fig. S2b–d†), which demonstrated the stability of the hybrid suspension. UV-vis absorption spectra of the hybrid suspension with different ratios are also shown in Fig. S5.† These results revealed that the absorption onset red shifted as the amount of GO in the suspension was increased and the maximum absorption intensity increased due to the presence of GO sheets, while the maximum absorption edge remained the same as that of the TO colloidal suspension. The zeta potential of GO (0.1 mg mL−1), TO (0.08 mg mL−1) as well as the GO (0.1 mg mL−1) and TO (0.08 mg mL−1) hybrid suspension (1
O.38,39 When mixing the two kinds of colloidal suspension, no obvious aggregates were observed, even after UV irradiation for different lengths of time (Fig. S2b–d†), which demonstrated the stability of the hybrid suspension. UV-vis absorption spectra of the hybrid suspension with different ratios are also shown in Fig. S5.† These results revealed that the absorption onset red shifted as the amount of GO in the suspension was increased and the maximum absorption intensity increased due to the presence of GO sheets, while the maximum absorption edge remained the same as that of the TO colloidal suspension. The zeta potential of GO (0.1 mg mL−1), TO (0.08 mg mL−1) as well as the GO (0.1 mg mL−1) and TO (0.08 mg mL−1) hybrid suspension (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) were −41 mV, −42.5 mV and −45.4 mV, respectively. These results demonstrated the excellent stability of GO and TO as well as the GO and TO hybrid suspensions.
1 in volume) were −41 mV, −42.5 mV and −45.4 mV, respectively. These results demonstrated the excellent stability of GO and TO as well as the GO and TO hybrid suspensions.
We investigated the photo-induced hydrophilicity of GO/TO hybrid films fabricated by the LBL as well as drop-casting methods based on different configurations, as shown in Fig. 2 and Fig. 3. The as-prepared GO/TO hybrid films fabricated via the LBL method were irradiated with UV light for 48 h to remove the PDDA layer. Then all the films were stored in the dark at 80 °C for 3 days. The hybrid films fabricated via the drop-casting method were examined directly after storage in the dark for 10 h due to their polymer-free nature. In Fig. 2a, with UV irradiation the contact angles of the TO-GO films fabricated by the drop-casting method increased at first and then slowly decreased after UV irradiation for 12 h. The insets show photographs of water drops deposited on the hybrid films after UV irradiation for different lengths of time, which clearly show the change in the contact angle. The mechanism of hybrophilicity modulation can be explained as follows: each individual GO sheet can be viewed as graphene decorated with oxygen functional groups on both sides of the sheet as well as around the edges.35 These functional groups make the GO sheets superhydrophilic. As UV irradiation continued, electron–hole pairs were generated in the TO nanosheets. The photo-generated electrons were directly captured by the GO sheets which were then reduced to rGO sheets. With further UV treatment, more electron–hole pairs were generated and the GO sheets captured more photo-generated electrons, leading to a greater extent of reduction, which represented the decrease in the amount of oxygen functional groups and greater π network restoration within the graphene structure. This modification resulted in an increase in the contact angle. However, longer UV irradiation led to a decrease in carbon content and an increase in carbon defects40 as well as the easier absorption of dissociated water on defects, which resulted in a decrease in contact angle. To further investigate the photo-induced hydrophobic/hydrophilic conversion of GO/TO hybrid films fabricated by the drop-casting method, we conducted the same experiment with GO–TO and GO/TO samples. In Fig. 2b, the contact angle decreased quickly with UV irradiation in an exponential form, and agreed well with previous results.21,22 As the TO layers were the topmost layer of the sample, upon UV irradiation, the change of the contact angle mostly reflected the properties of TO nanosheets. However, in Fig. S6,† where the sample was fabricated by drop-casting with GO/TO hybrid suspension as the source, surprisingly it was found that the contact angle remained nearly constant. This could be attributed to the fact that the GO and TO nanosheets self-assembled together in the suspension. When drop-cast onto the substrate, these hybrid films self-assembled at the interface of the substrates to form a nearly uniform arrangement with a GO layer on the top and a TO layer on the bottom. As most of the GO sheets were on the top, the hybrid films made by drop-casting exhibited the properties of GO sheets, whose contact angles increased slowly upon UV irradiation for a short period of time.
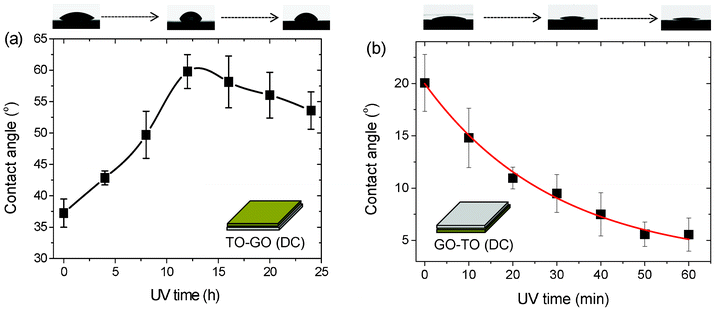 | ||
| Fig. 2 Photo-induced wettability evolution of (a) TO–GO and (b) GO–TO films formed by drop-casting. | ||
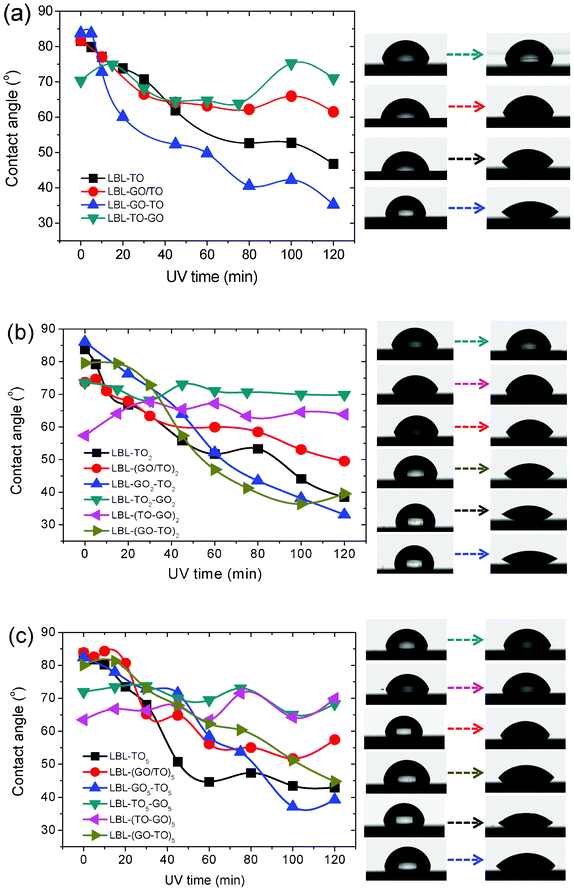 | ||
| Fig. 3 Photo-induced wettability evolution of different GO and TO hybrid structures formed by LBL. (a) n = 1. (b) n = 2. (c) n = 5. Right panels show the corresponding photographs of water droplets. | ||
As shown in Fig. 3, for samples fabricated via the LBL method, different structures exhibited different trends in the change in contact angle under UV irradiation. For structures of (GO/TO)n, GOn–TOn and (GO–TO)n, all the contact angles decreased with the increase of UV irradiation time. While for structures of TOn–GOn and (TO–GO)n, the contact angles fluctuated on a small scale. This could be explained as follows: for structures of (GO/TO)n, GOn–TOn and (GO–TO)n, the topmost layer is TO, whose contact angle decreased upon UV irradiation. While for structures of TOn–GOn and (TO–GO)n, GO sheets stayed on the top. After UV irradiation for 48 h, they were reduced thoroughly and all converted into relatively hydrophobic forms. A common phenomenon was demonstrated in Fig. 3: after UV irradiation for 120 min, the change of contact angle decreased in the order of rGOn–TOn, (rGO–TO)n and (rGO/TO)n and the time for reaching saturation also decreased in this order. As shown in Fig. 1e, the substrate coverage achieved via the LBL method from the GO/TO hybrid colloidal suspension was smaller than that of films made from only one component in suspension. The nanosheets on the substrate were more isolated from others and there were also fewer TO nanosheets, which led to the lowest change of contact angle under UV irradiation. However, there remained a question as to why the change of contact angle of GOn–TOn was larger than that of (GO–TO)n. Although the mechanism of photo-induced hydrophilic conversion of TO is still under discussion, two key explanations are proposed here: (i) photocatalytic decomposition of hydrophobic contaminates on the surface;41–43 (ii) structure changes of TO.44–49 In our cases, if the first explanation was responsible for the photo-induced hydrophilic conversion, according to the previous work,34 the photo-generated electrons would be quickly captured by the interlayer GO sheets between each two TO layers, which would prohibit the recombination of electron–hole pairs and lead to the enhancement of the photocatalytic capability of TO. Then (GO–TO)n would more effectively decompose hydrophobic contaminates on the surface than GOn–TOn. However, the results were opposite in our cases, which demonstrates that the second explanation seems more reasonable for the photo-induced hydrophilic conversion of TO. The change of contact angle of GOn–TOn was larger than that of (GO–TO)n, which could be attributed to the hypothesis that the GO sheets under the TO layers could promote structure changes in TO nanosheets thanks to the removal of the oxygen functional groups from the GO sheets during the photo reduction process, and thus more effective photo-induced hydrophilic conversion was obtained. The effect of GO on the structure of the TO nanosheets needs to be further investigated.
Upon UV irradiation, there was a tendency for GO sheets to exhibit an increase in contact angle. In other words, with UV treatment, the surface of a GO sheet changed from hydrophilic to relatively hydrophobic. As the TO nanosheets exhibited the reverse tendency of surface hydrophilicity, we could imagine that, after hybridization of GO and TO nanosheets, the two surfaces of the hybrid films could achieve relatively different hydrophilic/hydrophobic properties upon UV irradiation, which will have potential future applications in water transport engineering.
Based on the above idea, freestanding hybrid films of GO and TO sheets were fabricated, as illustrated in Fig. 4a: first the GO suspension was drop-cast on a piece of paper, followed by drying in air, then the TO suspension was drop-cast on the formed GO film followed by drying under the same conditions. The as-prepared freestanding hybrid film shows excellent flexibility, mechanical strength and a similar contact angle changing tendency to that in Fig. 2: the GO surface changed from hydrophilic to relatively hydrophobic upon UV irradiation, while the TO surface showed the reverse tendency. The difference in wettability of both surfaces of the hybrid film can be tuned by UV irradiation time (Fig. 4b and Fig. 4c). To demonstrate the ability of water collection of both surfaces of the freestanding hybrid film, after 8 h UV irradiation the freestanding film was moisturized by an air humidifier. As shown in Fig. 4d and Fig. 4e, lots of condensed water droplets were observed on the TO surface, revealing its excellent hydrophilic nature. On the contrary, many fewer water drops were formed on the reduced GO surface. The above phenomena demonstrated the excellent dual photo-induced tunable wettability of GO and TO hybrid films, which will have potential future applications in water collection and transportation.
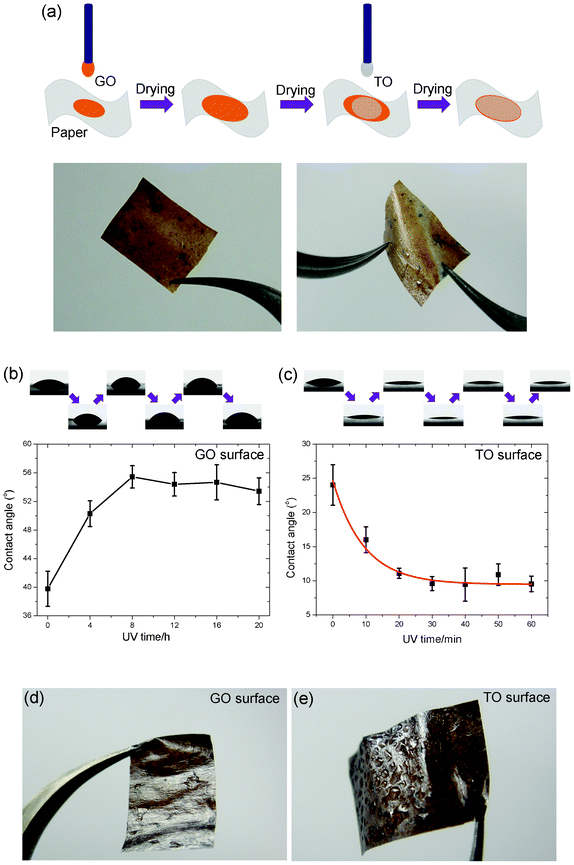 | ||
| Fig. 4 (a) Process schematics for fabricating the freestanding hybrid film of GO and TO sheets, and corresponding photographs (b) and (c) Dual tunable hydrophilicity of the hybrid film under UV irradiation. (d) and (e) Photographs of two UV treated surfaces of the humidified film. | ||
4. Conclusions
In conclusion, we have fabricated GO and TO hybrid films via the LBL and drop-casting methods and investigated the decomposition of PDDA and GO reduction in lamellar films fabricated via the LBL method. We investigated the photo-induced wettability evolution of the hybrid films with different configurations. In the hybrid films of GO and TO, the GO sheets exhibited photo-induced hydrophobic conversion upon UV irradiation, while TO nanosheets showed the reverse trend. After hybridization, both sides of the film show opposite surface property change tendencies under UV irradiation. The hybrid films of GO and TO nanosheets will have potential future applications in liquid transport engineering.Experimental section
GO preparation
Natural graphite flakes were mixed with concentrated sulfuric acid and hydrogen peroxide, after that the mixture was stirred for 1 h and then washed with deionized (DI) water until the pH reached 7. After drying at 40 °C for 24 h, the obtained graphite intercalation compounds were converted to expanded graphite through fast heating at 900 °C for 10 s. Then the obtained worm-like graphite was further treated with a modified Hummer's method to obtain graphite oxides. The GO colloidal suspension was further obtained by sonication in water. The fabrication processes of the hybrid samples by LBL and DC methods are illustrated in Fig. S1.†Microscopic characterizations
The photographs of the as-prepared GO (0.1 mg mL−1) and TO (0.08 mg mL−1) colloidal suspensions are shown in Fig. S2.† Both of these colloidal suspensions exhibit a clear, uniform nature and show no obvious agglomerates after several weeks. Fig. S3a† shows the TEM image of GO sheets, which possess a lateral size in the magnitude of micrometers. Fig. S3b† shows the TEM image of TO nanosheets, which possess a lateral size of several hundred nanometers and distribute uniformly on the TEM grid. Fig. S3c† shows a TEM image of the GO–TO hybrid film assembled by the LBL method, clearly showing two layers of nanosheets. Those with larger lateral sizes are the GO sheets while the ones with smaller lateral sizes are the TO nanosheets. Fig. S3d† is a TEM image of the GO/TO hybrid film fabricated by drop-casting using the GO and TO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) colloidal mixture suspension as the source. All the nanosheets pack together and it is hard to distinguish between the two different kinds of sheets.
1 in volume) colloidal mixture suspension as the source. All the nanosheets pack together and it is hard to distinguish between the two different kinds of sheets.
XRD
The interlayer spacing changes of the hybrid samples were revealed by XRD, as shown in Fig. S4a and S4b.† The diffraction peaks of as-prepared hybrid films of GOn–TOn and (GO–TO)n are located at 2θ = 5° and 4.98°, respectively. While after UV irradiation for 24 h, the diffraction peaks shift to 2θ = 6.92° and 5.98°, corresponding to a decrease of the interlayer distance from 1.77 nm to 1.28 nm and 1.48 nm, respectively. After UV irradiation for 48 h, the diffraction peaks further shift to 2θ = 7.46° and 6.8°, corresponding to a further decrease of the interlayer distance to 1.18 nm and 1.30 nm, respectively. Both of as-prepared samples have been subjected to annealing at 300 °C for 1 h instead of UV irradiation, and they show a diffraction peak at 2θ = 8.5°, corresponding to an interlayer distance of 1.04 nm. Based on above results, we propose a mechanism to explain the structural evolution of GOn–TOn and (GO–TO)n samples under UV irradiation, as shown in Fig. S4c and S4d.† Upon UV irradiation, GOn–TOn and (GO–TO)n samples showed different procedures of PDDA decomposition along with reduction of GO to rGO. In (GO–TO)n, UV generated holes and electrons in the titania layers were used for PDDA decomposition and the reduction of GO to rGO and these two processes occurred simultaneously, resulting in a relatively slow and uniform decrease in the interlayer distance. As for the sample of GOn–TOn, the PDDA layers beneath the TO layers decomposed faster than those beneath the GO layers and hence GO layers were reduced much more slowly, resulting in a sharper decrease in the interlayer distance of the titania layers on the top than that in (GO–TO)n. As the decomposition of the PDDA layers beneath the TO layers was almost complete, the decomposition of PDDA layers beneath GO sheets as well as the reduction of GO to rGO became faster. The nearly identical interlayer spacing of TO layers on the top as well as the sharp decrease in the interlayer spacing of GO layers resulted in a relatively slower decrease in the interlayer spacing than that in (GO–TO)n. Finally, after further UV irradiation, GOn–TOn and (GO–TO)n samples reached nearly the same interlayer spacing.UV-vis absorption spectra
We have also collected UV-vis absorption spectra for the samples fabricated by drop-casting and the LBL method as well as liquid phase hybrid samples. In Fig. S5a,† GO sheets drop-cast on quartz glass exhibit an absorption peak at ∼225 nm, which is dominated by π–π* transitions. While other GO (rGO)/TO hybrid films exhibit two absorption peaks located at ∼225 nm and ∼265 nm, which are characteristic of GO and TO nanosheets, respectively. After UV irradiation for 48 h, due to the reduction of GO to rGO, both the GO–TO and GO/TO hybrid films show stronger light absorption. The UV-vis absorption spectra of the samples fabricated by the LBL method are shown in Fig. S5b,† in all the samples exists an intense absorption peak at ∼265 nm and a weak shoulder at ∼225 nm. For different stacking sequences of GO and TO nanosheets, as the number of layers increases, the peak intensity located at ∼265 nm as well as the shoulder located at ∼225 nm increase gradually, suggesting a uniform configuration of GO and TO nanosheets. It is interesting to note that after 48 h of UV irradiation, the peak intensities of these two different structures change differently. For GOn–TOn (n = 1, 2, 5), the peak intensity located at ∼265 nm and the shoulder located at ∼225 nm increase after UV treatment, while for (GO–TO)n (n = 1, 2, 5), the peak and the shoulder decrease after UV irradiation for 48 h (Fig. S5d,e).†Acknowledgements
This work was supported by the National Science Foundation of China (50972067) and the Beijing Natural Science Foundation (2122027).References
- X. Deng, L. Mammen, H. J. Butt and D. Vollmer, Science, 2012, 335, 67 CrossRef CAS.
- J. Rafiee, X. Mi, H. Gullapalli, A. V. Thomas, F. Yavari, Y. F. Shi, P. M. Ajayan and N. A. Koratkar, Nat. Mater., 2012, 11, 217 CrossRef CAS.
- M. K. Chaudhury and G. M. Whitesides, Science, 1992, 256, 1539 CAS.
- A. M. Cazabat, F. Heslot, S. M. Troian and P. Caries, Nature, 1990, 346, 824 CrossRef CAS.
- A. Quinn, R. Sedev and J. Ralston, J. Phys. Chem. B, 2003, 107, 1163 CrossRef CAS.
- V. Peykov, A. Quinn and J. Ralston, Colloid Polym. Sci., 2000, 278, 789 CAS.
- M. Schneemilch, W. J. J. Welters, R. A. Hayes and J. Ralston, Langmuir, 2000, 16, 2924 CrossRef CAS.
- S. Abbott, J. Ralston, G. Reynolds and R. Hayes, Langmuir, 1999, 15, 8923 CrossRef CAS.
- N. Stevens, C. I. Priest, R. Sedev and J. Ralston, Langmuir, 2003, 19, 3272 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Adv. Mater., 1998, 10, 135 CrossRef CAS.
- N. Sakai, R. Wang, A. Fujishima, T. Watanabe and K. Hashimoto, Langmuir, 1998, 14, 5918 CrossRef CAS.
- R. Wang, N. Sakai, A. Fujishima, T. Watanabe and K. Hashimoto, J. Phys. Chem. B, 1999, 103, 2188 CrossRef CAS.
- T. Watanabe, A. Nakajima, R. Wang, M. Minabe, S. Koizumi, A. Fujishima and K. Hashimoto, Thin Solid Films, 1999, 351, 260 CrossRef CAS.
- T. Tanaka, Y. Ebina, K. Takada, K. Kurashima and T. Sasaki, Chem. Mater., 2003, 15, 3564 CrossRef CAS.
- T. Sasaki, M. Watanabe, H. Hashizume, H. Yamada and H. Nakazawa, J. Am. Chem. Soc., 1996, 118, 8329 CrossRef CAS.
- T. Sasaki, Y. Ebina, T. Tanaka, M. Harada, M. Watanabe and G. Decher, Chem. Mater., 2001, 13, 4661 CrossRef CAS.
- T. Sasaki, Y. Ebina, M. Watanabe and G. Decher, Chem. Commun., 2000, 2163 RSC.
- M. Osada, Y. Ebina, H. Funakubo, S. Yokoyama, T. Kiguchi, K. Takada and T. Sasaki, Adv. Mater., 2006, 18, 1023 CrossRef CAS.
- K. Akatsuka, M. Haga, Y. Ebina, M. Osada, K. Fukuda and T. Sasaki, ACS Nano, 2009, 3, 1097 CrossRef CAS.
- N. Sakai, K. Fukuda, T. Shibata, Y. Ebina, K. Takada and T. Sasaki, J. Phys. Chem. B, 2006, 110, 6198 CrossRef CAS.
- T. Shibata, N. Sakai, K. Fukuda, Y. Ebina and T. Sasaki, Mater. Sci. Eng., B, 2009, 161, 12 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. S. Zhang, V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS.
- Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS.
- C. Gómez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499 CrossRef.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394 CrossRef CAS.
- X. Wang, L. Zhi and K. Müllen, Nano Lett., 2008, 8, 323 CrossRef CAS.
- H. A. Becerill, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463 CrossRef.
- G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487 CrossRef CAS.
- O. Akhavan and E. Ghaderi, J. Phys. Chem. C, 2009, 113, 20214 CAS.
- K. K. Manga, Y. Zhou, Y. Yan and K. P. Loh, Adv. Funct. Mater., 2009, 19, 3638 CrossRef CAS.
- H. C. Schniepp, J. L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535 CrossRef CAS.
- W. Gu, W. Zhang, X. Li, H. Zhu, J. Wei, Z. Li, Q. Shu, C. Wang, K. Wang, W. Shen, F. Kang and D. Wu, J. Mater. Chem., 2009, 19, 3367 RSC.
- J. Robertson, Mater. Sci. Eng., R, 2002, 37, 129 CrossRef.
- J. I. Paredes, S. Villar-Rodil, P. Solı ́s-Fernández, A. Martı ́nez-Alonso and J. M. D. Tascón, Langmuir, 2009, 25, 5957 CrossRef CAS.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- O. Akhavan, M. Abdolahad, A. Esfandiar and M. Mohatashamifar, J. Phys. Chem. C, 2010, 114, 12955 CAS.
- C.-Y. Wang, H. Groenzin and M. J. Shultz, Langmuir, 2003, 19, 7330 CrossRef CAS.
- J. M. White, J. Szanyi and M. A. Henderson, J. Phys. Chem. B, 2003, 107, 9029 CrossRef CAS.
- T. Zubkov, D. Stahl, T. L. Thompson, D. Panayotov, O. Diwald and J. T. Jr. Yates, J. Phys. Chem. B, 2005, 109, 15454 CrossRef CAS.
- N. Sakai, A. Fujishima, T. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2001, 105, 3023 CrossRef CAS.
- N. Sakai, A. Fujishima, T. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 1028 CrossRef CAS.
- R. Nakamura, K. Ueda and S. Sato, Langmuir, 2001, 17, 2298 CrossRef CAS.
- A. Y. Nosaka, E. Kojima, T. Fujiwara, H. Yagi, H. Akutsu and Y. Nosaka, J. Phys. Chem. B, 2003, 107, 12042 CrossRef CAS.
- A. Y. Nosaka and Y. Nosaka, Bull. Chem. Soc. Jpn., 2005, 78, 1595 CrossRef CAS.
- K. Uosaki, T. Yano and S. Nihonyanagi, J. Phys. Chem. B, 2004, 108, 19086 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21699j |
| This journal is © The Royal Society of Chemistry 2012 |
