A multifunctional magnetic hybrid synthesized for adsorption of environmental contaminants†
Hong-Wen
Gao
*,
Yang
Cui
,
Yan-Ping
Wei
,
Gang
Xu
and
Zhang-Jun
Hu
State Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai, 200092, China. E-mail: EMSL@tongji.edu.cn; Fax: (+) 86-21-65988598
First published on 17th September 2012
Abstract
A magnetic hybrid was synthesized by self-assembly of ferromagnetic oxide particles into layered magnesium silicate using octadecylamino as a soft template. It adsorbed lipophilic pesticides from aqueous solution then could be calcined and reused as a porous magnetic sorbent for remediation of water contaminated by heavy metals.
In the last decade, the wanton depletion of resources and discharge of industrial waste e.g. pesticides and heavy metals, seriously threaten the living environment of mankind, especially in developing countries.1 Environmentally functional materials play an increasingly important role in the elimination of contaminants. Studies concerning these materials focus on facile preparation, efficient elimination of toxic contaminants, easy separation and reuse of waste residue.2 In recent years, inorganic–organic (IO) materials with complex hierarchical structures synthesized through “host–guest” chemistry have been successfully employed in the field of environmental science,3 particularly as sorbents for water remediation.4 Some entity frameworks in layered architectures are utilized as ideal “host” supports in the construction of multifunctional sorbents e.g. layered silicates,5 layered double hydroxides,6 layered transition metal oxides,7 graphitic carbon and other lamellar materials.8 Among the potential “guest” molecules, surfactants have attracted much attention due to their hydrophobic and electric properties, used as adsorptive moieties and steerable arrays for soft templates.9 The prepared hybrid materials have to be washed repeatedly with organic solvents or calcined at high temperature to remove the templates.10 This causes waste surfactants and environmental pollution. Although exhibiting good adsorptive properties, most hybrid materials usually exist as an ultrafine powder and they are difficult to separate from aqueous solution. Magnetic hybrid materials have been technologically exploited due to their easy separation in an external magnetic field.9b,10c,11 Therefore, using a facile approach involving templating and self-assembly of layered building blocks to prepare a reusable magnet-based material is attractive and challenging.
Organic groups in a hierarchical IO sorbent can be easily fabricated, but it is difficult for an inorganic structure to be prepared in a controlled manner simultaneously. A number of eco-friendly IO sorbents have been reported for dye contaminant adsorption through “host–guest” chemistry, as well as for the reuse of the waste sludge.6b,8c However, only limited types of contaminants were adsorbed. As mentioned above, a proper template can work not only as adsorptive moiety but also as a pillar to build multiscale porosity in the framework of the IO hybrid. The obtained porosity could be used to adsorb other pollutants after the template is removed from the initial sorbent. If the combination involved in the synthetic process works well, the remediation of two or more types of contaminated waters may be achieved, e.g. organic pollutants and heavy metals. Also, magnetic separation can provide a convenient method to recycle the sorbent. This constructive protocol will increase the usage of resources and simultaneously reduce pollution, in accordance with the perception of “using waste to treat waste”.
A facile synthesis of magnetic hybrid was carried out by self-assembly of ferromagnetic oxide particles (FOP) into layered magnesium silicate using an octadecylamino (OA) surfactant as a soft template. The hybrid named FOASH has a high sorption capacity to lipophilic pesticides. From infrared spectra (IR) in Fig. 1A, the Mg–O/Fe–O and Si–O absorption peaks are located at 460 and 1020 cm−1, respectively. The absorption peaks at 3450, 2920, 2850 and 1470 cm−1 indicate that OA is embedded successfully into FOASH. By IR comparison of OA,5c a new absorption peak of FOASH appeared at 1270 cm−1 and it may be due to hydrogen bonds formed between OA and FOP and Mg–O–Si layer. After FOASH was calcined at 600 °C, the OA absorption peaks disappeared (Fig. 1A), i.e. OA was completely decomposed. The Fe–O peak at 540 cm−1 appears and the Si–O peak shifts to 1090 cm−1. The hybrid material was further confirmed by thermal gravimetric analysis (TGA). The total weight loss of FOASH is approximately 60% before 500 °C (Fig. 1B). The decomposition of OA occurred between 180 and 500 °C. Thus, approximately 45% OA was embedded in FOASH. The Mg, Si and Fe oxides were formed after 500 °C (Fig. 1B(2)). The surface area of FOASH was measured to be only 66 m2 g−1, where nano pores that are <100 nm occupy 36 m2 g−1 (Fig. 1C(1)). Pores that are <20 nm own over 40% of the pore area. Embedding OA doesn't obviously increase the surface area of material. After FOASH was calcined, the surface area of metal oxides composite (CMOC) material increased to 123 m2 g−1, where nano pores occupy 44 m2 g−1. Owing to the decomposition of OA, 80% of the surface area was contributed by pores <5 nm (Fig. 1C(2)) and 50% of that by pores <2 nm. It is favourable for filling with metal ions and little molecules.
 | ||
| Fig. 1 A: IR spectra of the FOASH (1) and CMOC (2) materials, B: 1 – TG change of FOASH and 2 – its derivative weight change (2). C: Distribution of the cumulative surface area for nano pores (<100 nm) in the FOASH (1) and CMOC (2) materials. | ||
From TEM and small-angle X-ray diffraction (SAXRD) of FOASH (Fig. 2A(a)) (Fig. S1, ESI†), plenty of toroidal layer-by-layer structures were clearly observed. It indicates that the OA layer and Mg–O–Si layer extend alternately outward around FOP. Because FOP and Mg–O–Si particles are negatively charged, the positively charged OA binds to FOP and Mg–O–Si layers via electric charge attraction.12 The hydroxyethyl group of OA may further interact with the metal oxides via hydrogen bond. The OA bilayer intercalation structure is formed by the tail-to-tail hydrophobic stack with the interval of 4.8 nm (Fig. 2A(b)) (Fig. S1 D, ESI†).
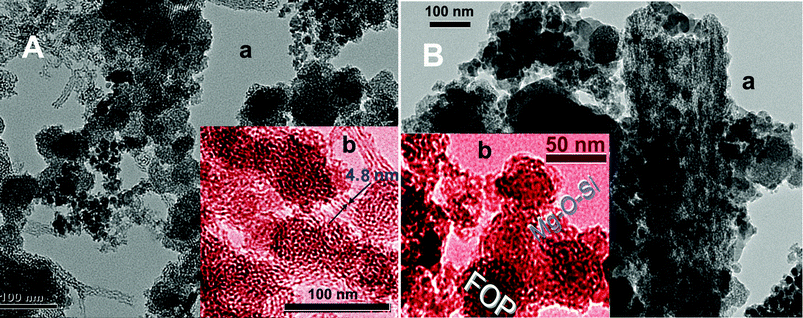 | ||
| Fig. 2 TEM images of FOASH (A) and CMOC (B), where images b are colored for convenient observation. | ||
After calcining, the CMOC material becomes porous with a honeycomb structure and large numbers of extremely little nano pores left (Fig. 2B(a)) (Fig. S2, ESI†). Their formation is due to the OA decomposition gas escaping through the Mg–O–Si layer at high temperature. In the CMOC aggregation, the Mg–O–Si layers collapsed into the clathrate structure and enclosed FOP (Fig. 2B(b)). The surface area of the pores increased, which was confirmed by the cumulative surface area (Fig. 1C).
The saturation magnetization (Ms) of CMOC approaches 12 emu/g, which is comparable to Ms of FOASH (14 emu/g) (Fig. 3A). Thus, the CMOC material remains magnetic so as to conveniently separate. From TGA curves and TEM images (Fig. 1B, 2A), plenty of OA bilayers were intercalated in FOASH, which may cause a high sorption capacity to lipophilic organic chemicals. As a representative experiment, the sorption of two common organo-phosphorus pesticides: parathion-methyl and phoxim (Fig. 3B(b)) was carried out using FOASH and their residue determined (Fig. S3, ESI†). Their sorptions were in accordance with the lipid-water partition (Fig. 3B(a)) and the partition coefficients (Kaw) were calculated to be 3.38 × 103 and 2.61 × 104 L kg−1, respectively. 0.1% (wt%) FOASH removed 75% parathion-methyl and 95% phoxim (Fig. 3B(b)), being superior to activated carbon.13 It is impossible for sorption of these chemicals to depend on the little surface area of FOASH (Fig. 1C). They may dissolve into the OA bilayer via hydrophobic interaction.5b,9b The spent FOASH was separated readily with a rod magnet (Fig. 3A(a)).
 | ||
| Fig. 3 The hysteresis curves of the FOASH (1) and CMOC (2) materials (A), a – the pesticide–FOASH mixing liquid (light yellow test-tube) and its separation with a magnet rod (clear); b – the heavy metals-CMOC mixing liquid (yellow) and its separation (clear). The parathion-methyl (1) and phoxim (2) solutions were adsorbed, respectively by 0.02% (wt%) FOASH (B), a – plots qevs. ce (qe – sorption amount of pesticides and ce – their equilibrium concentrations), respectively by 0.02% (wt%) FOASH; b – chromatographic analysis of a solution containing 5.0 mg L−1 parathion-methyl (1) and 4.0 mg L−1 phoxim (2), where the color areas show the sorption of pesticides to 0.1% FOASH. C – The heavy metals solutions (1 and 3) were treated with 0.5% (wt%) CMOC (2 and 4). | ||
In order to avoid secondary pollution and increase the comprehensive use of resources, recycling of spent materials is often necessary.9b,14 When the FOASH sludge was calcined at 600 °C, all organic compounds i.e. OA and pesticides were decomposed (Fig. 1A, B). Plenty of pores <5 nm remained (Fig. 1C, 2B) and they are favourable for filling with heavy metals ions. Two solutions mixed with five kinds of heavy metal ions, i.e. Cu2+, Cd2+, Zn2+, Pb2+ and Cr3+ were treated with only 0.5% (wt%) CMOC. From the difference between columns 3 and 4 in Fig. 3C, >99.5% of heavy metals are removed from the solution containing each metal in 10 mg L−1. In the other solution containing each metal in 50 mg L−1, the removal rates of Pb2+, Cr3+ and Cu2+ are >95% by comparing columns 2 with 1. The sorption capacity of CMOC to heavy metals is estimated to be approximately 40 mg g−1, which is obviously higher than ones of other waste residues, natural sorbents and even artificially synthesized materials.15 In addition, the acidity (pH 2.92 to 5.96) of raw solutions seldom affected the removal rate of heavy metals (Fig. S4, ESI†). Heavy metal ions may fill into nano pores to coordinate with silicon, magnesium and iron oxides. The CMOC sludge capturing heavy metals remains magnetic (Fig. 3A(b)).
In conclusion, OA and silicate grew alternately around FOP. The layered magnetic material adsorbed strongly with lipophilic organic chemicals. The CMOC exhibited an outstanding sorption capacity to heavy metals. The FOASH material plays a multifunctional role in elimination of water contamination and reusability of resources. In addition, the material can be readily prepared and easily separated from aqueous solution. Prospectively, the CMOC sludge may be further treated to extract heavy metals for industrial use.
Acknowledgements
The authors acknowledge a financial support from the Foundation of State Key Laboratory of Pollution Control and Resource Reuse, China (Grant No. PCRRK11003) and the Natural Science Foundation of China (No.21007046).References
- (a) P. Webster, Science, 2004, 306, 1875 CrossRef CAS; (b) Q. Zhang, Z. Li, G. Zeng, J. Li, Y. Fang, Q. Yuan, Y. Wang and F. Ye, Environ. Monit. Assess., 2009, 152, 123 CrossRef CAS; (c) L. Guo, Science, 2007, 317, 1166 CrossRef CAS.
- (a) W. Liu, F. Huang, Y. Liao, J. Zhang, G. Ren, Z. Zhuang, J. Zhen, Z. Lin and C. Wang, Angew. Chem., Int. Ed., 2008, 47, 5619 CrossRef CAS; (b) A. Tal, Science, 2007, 316, 1698 CAS.
- (a) A. Corma, U. Diaz, T. Garcia, G. Sastre and A. Velty, J. Am. Chem. Soc., 2010, 132, 15011 CrossRef CAS; (b) D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. M. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587 CrossRef CAS; (c) N. Takahashi, H. Hata and K. Kuroda, Chem. Mater., 2010, 22, 3340 CrossRef CAS.
- (a) K. H. Goh, T. T. Lim and Z. L. Dong, Environ. Sci. Technol., 2009, 43, 2537 CrossRef CAS; (b) S. J. Lee, S. S. Lee, M. S. Lah, J. M. Hong and J. H. Jung, Chem. Commun., 2006, 42, 4539 RSC.
- (a) D. Mochizuki, A. Shimojima, T. Imagawa and K. Kuroda, J. Am. Chem. Soc., 2005, 127, 7183 CrossRef CAS; (b) A. Monnier, F. Schüth, Q. Huo, D. Kumar, D. Margolese, R. S. Maxwell, G. D. Stucky, M. Krishnamurty, P. Petroff, A. Firouzi, M. Janicke and B. F. Chmelka, Science, 1993, 261, 1299 CAS; (c) Y. P. Wei and H. W. Gao, J. Mater. Chem., 2012, 22, 5715 RSC.
- (a) Y. F. Xu, Y. C. Dai, J. Z. Zhou, Z. P. Xu, G. R. Qian and G. Q. M. Lu, J. Mater. Chem., 2010, 20, 4684 RSC; (b) Y. P. Wei, D. Q. Wei and H. W. Gao, Chem. Eng. J., 2011, 172, 872 CrossRef CAS.
- S. N. Britvin, A. Lotnyk, L. Kienle, S. V. Krivovichev and W. Depmeier, J. Am. Chem. Soc., 2011, 133, 9516 CrossRef CAS.
- (a) M. V. Jimenez, M. Algarra, J. J. Jimenez and M. Lamotte, Chemosphere, 2004, 57, 179 CrossRef CAS; (b) M. J. Manos, V. G. Petkov and M. G. Kanatzidis, Adv. Funct. Mater., 2009, 19, 1087 CrossRef CAS; (c) D. H. Zhao, Y. L. Zhang, Y. P. Wei and H. W. Gao, J. Mater. Chem., 2009, 19, 7239 RSC.
- (a) C. Boissiere, D. Grosso, A. Chaumonnot, L. Nicole and C. Sanchez, Adv. Mater., 2011, 23, 599 CrossRef CAS; (b) P. Wang, Q. H. Shi, Y. F. Shi, K. K. Clark, G. D. Stucky and A. A. Keller, J. Am. Chem. Soc., 2009, 131, 182 CrossRef CAS; (c) J. Lin and H. W. Gao, J. Mater. Chem., 2009, 19, 3598 RSC.
- (a) S. J. Wu, F. T. Li, Y. N. Wu, R. Xu and G. T. Li, Chem. Commun., 2010, 46, 1694 RSC; (b) J. Y. Xiao and L. M. Qi, Nanoscale, 2011, 3, 1383 RSC; (c) S. H. Tolbert, A. Firouzi, G. D. Stucky and B. F. Chmelka, Science, 1997, 278, 264 CrossRef CAS.
- (a) L. Li, Y. Feng, Y. Li, W. Zhao and J. Shi, Angew. Chem., Int. Ed., 2009, 48, 5888 CrossRef CAS; (b) A. Gorschinski, G. Khelashvili, D. Schild, W. Habicht, R. Brand, M. Ghafari, H. Bonnemann, E. Dinjus and S. Behrens, J. Mater. Chem., 2009, 19, 8829 RSC.
- (a) C. J. Meledandri, J. K. Stolarczyk and D. F. Brougham, ACS Nano, 2011, 5, 1747 CrossRef CAS; (b) H. W. Gao and X. H. Xu, Chem. Commun., 2011, 47, 12810 RSC.
- (a) V. K. Gupta, B. Gupta, A. Rastogi, S. Agarwal and A. Nayak, Water Res., 2011, 45, 4047 CrossRef CAS; (b) Q. L. Li, V. L. Snoeyink, B. J. Marinas and C. Campos, Water Res., 2003, 37, 4863 CrossRef CAS.
- Z. H. Sun, L. F. Wang, P. P. Liu, S. C. Wang, B. Sun, D. Z. Jiang and F. S. Xiao, Adv. Mater., 2006, 18, 1968 CrossRef CAS.
- (a) S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, Water Res., 1999, 33, 2469 CrossRef CAS; (b) P. Miretzky and A. F. Cirelli, J. Hazard. Mater., 2010, 180, 1 CrossRef CAS; (c) X. Tian, S. Zhou, Z. Zhang, X. He, M. Yu and D. Lin, Environ. Sci. Technol., 2010, 44, 8144 CrossRef CAS; (d) D. Yang, B. Paul, W. Xu, Y. Yuan, E. Liu, X. Ke, R. M. Wellard, C. Guo, Y. Xu, Y. Sun and H. Zhu, Water Res., 2010, 44, 741 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and Fig. S1 to S4. See DOI: 10.1039/c2ra20992f |
| This journal is © The Royal Society of Chemistry 2012 |
