Monomeric, not tetrameric species are responsible for the colossal dielectric constant of copper phthalocyanine derived from pyromellitic dianhydride†
Gellert
Mezei
*,
Andre R.
Venter
,
Joseph W.
Kreft
,
Alexander A.
Urech
and
Nicole R.
Mouch
Department of Chemistry, Western Michigan University, Kalamazoo, MI 49008, USA. E-mail: gellert.mezei@wmich.edu; Tel: 1-269-387-2859
First published on 6th September 2012
Abstract
Mass spectrometric analysis indicates that the copper(II) phthalocyanine derived from pyromellitic dianhydride is a mononuclear species, not a tetranuclear species as previously thought. The peripheral substitution of the phthalocyanine core with eight carboxylic acid groups, rather than extended conjugation, is responsible for the extraordinary dielectric constant associated with this material.
In our endeavor to prepare inexpensive, robust organic solar cells,1 we recently focused our attention on a tetranuclear copper(II) phthalocyanine (CuPc) “oligomer”, (Cu4Pc*)(COOH)16 (Scheme 1), reported to possess an extremely high dielectric constant2 and large electromechanical responses in organic polymer composites.3 Materials with such properties are highly sought after for applications in electromechanical actuators, artificial muscles, smart skins for drag reduction, microfluidic systems for drug delivery, and high efficiency charge storage capacitors.2–4 A closer examination of the reported preparation and characterization of (Cu4Pc*)(COOH)16, followed by a thorough analysis of the literature, revealed a lack of direct evidence of the structure of this rather unusual molecule, and a general confusion in referring to monomeric, oligomeric and polymeric phthalocyanines. A large number of publications† report properties and conclusions based on the assumed structure of (Cu4Pc*)(COOH)16, which was proposed based on infrared spectroscopy, elemental analysis and titrimetry three decades ago.5 Several composite materials containing “phthalocyanine tetramers” mixed with3,6 or grafted onto organic polymers7 and nanoparticles8 were also reported. Therefore, we considered it imperative to re-examine the identity of this copper complex obtained from 1,2,4,5-tetracarboxylic (pyromellitic) acid derivatives. Herein, we provide a summary of the history of the reaction of pyromellitic acid derivatives with urea and metal salts,† and present for the first time a mass spectrometric analysis of the materials obtained from pyromellitic dianhydride (PMDA) and MSO4 (M = Cu and Co), as well as of the C12-imide functionalized copper derivative.
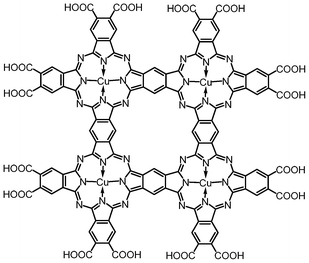 | ||
| Scheme 1 Structure of the proposed cyclic, fused phthalocyanine tetramer, (Cu4Pc*)(COOH)16. | ||
The reaction of PMDA, urea, NH4Cl, metal salt and ammonium molybdate in nitrobenzene at 180–185 °C was reproduced according to the published procedure (including the purification steps by aqueous NaOH and HCl treatments).5 The resulting products consist of (MPc)(COOH)8 (M = Cu, 1a; M = Co, 1b). The same procedure was also employed in an alternative preparation of 1a, using refluxing N-methyl-2-pyrrolidone (NMP, b.p. = 202 °C) as the solvent instead of nitrobenzene. The UV-visible spectra display absorptions characteristic of the phthalocyanine ring9 at 350, 617 and 686 nm (1a), and 345, 614 and 683 nm (1b), respectively. The dielectric constant measured for 1a10 is virtually the same as the value reported for the assumed “tetramer”.11 Size-exclusion chromatography experiments with 1a showed that the whole sample, applied to Sephadex G-15, G-25 or G100 columns, elutes as a single band with 6 mM aqueous NH3 solution (pH = 10.5), indicating the presence of a single major component.
The negative mode electrospray ionization mass spectrum (ESI-MS) of 1a (Fig. 1) in N,N-dimethylformamide (DMF) displays numerous peaks across the m/z = 350–1860 region, which all originate from the mononuclear species (CuPc)(COOH)8 (MW = 928.15).† No peaks were detected at higher m/z values (up to m/z = 4000).
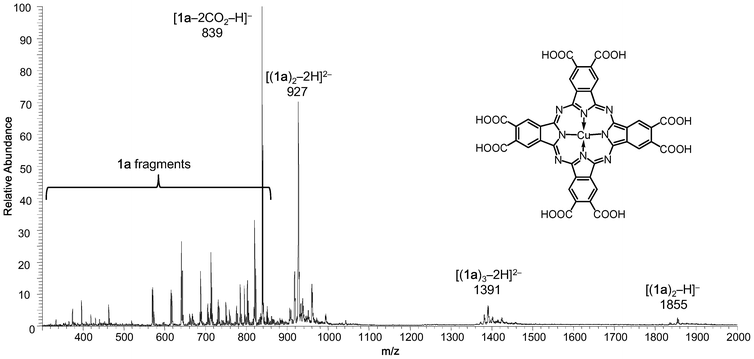 | ||
| Fig. 1 Negative mode ESI mass spectrum of 1a, showing peaks assignable to (CuPc)(COOH)8. | ||
In the m/z = 1830–1860 region, two minor peaks were identified and assigned to the singly deprotonated, stacked dimer [(1a)2–H]− (m/z = 1855, Figure S1†) and [(1a)2–H2O–H]− (m/z = 1837), respectively. The corresponding doubly deprotonated species, [(1a)2–2H]2− and [(1a)2–H2O–2H]2−, were observed at m/z = 927 (Figure S2†) and 918, respectively. The m/z = 1350–1450 region shows several minor peaks with isotopic patterns consistent with a 2− charged Cu3 species. These peaks were assigned to the doubly deprotonated stacked trimer [(1a)3–2H]2− (m/z = 1391, Figure S3†) and its dehydrated products [(1a)3–nH2O–2H]2− (n = 1, 2, 3).
All peaks in the m/z = 350–850 region were assignable to either singly or doubly deprotonated fragments of 1a, due to decarboxylation and/or dehydration: [1a–2CO2–H]− (m/z = 839, base peak, Figure S4†), [1a–2CO2–H2O–H]− (m/z = 821), [1a–2CO2–2H2O–H]− (m/z = 803), [1a–3CO2–H]− (m/z = 795), [1a–2CO2–3H2O–H]− (m/z = 785), [1a–3CO2–H2O–H]− (m/z = 777), [1a–3CO2–2H2O–H]− (m/z = 759), [1a–4CO2–H]− (m/z = 751), [1a–4CO2–H2O–H]− (m/z = 733), [1a–4CO2–2H2O–H]− (m/z = 715), [1a–5CO2–H]− (m/z = 707), [1a–5CO2–H2O–H]− (m/z = 689), [1a–6CO2–H2O–H]− (m/z = 645), [1a–7CO2–H]− (m/z = 619), [1a–2H]2− (m/z = 463), [1a–2CO2–2H]2− (m/z = 419), [1a–3CO2–2H]2− (m/z = 397), [1a–4CO2–2H]2− (m/z = 375).
The use of refluxing NMP instead of nitrobenzene in the synthesis of 1a resulted in a similar product, with virtually the same mass spectrum. Again, only the mononuclear species was observed in the ESI-MS of the corresponding Co analogue, 1b: [1b–H]− (m/z = 922), [1b+DMF–H]− (m/z = 995), [1b+2DMF–H]− (m/z = 1068), [1b–2CO2–H]− (m/z = 834), [1b–2H]2− (m/z = 461), [1b+DMF–2H]2− (m/z = 497), [1b–2CO2–2H]2− (m/z = 417).
Further proof of the identity of 1a came from chemical derivatization, upon transformation of the dicarboxylic acid units into dodecyl-imide groups (2, Scheme 2).‡ The added C12 groups led to a complete reversal of the compound's polarity, so that while the carboxylic acid starting material is only soluble in highly polar solvents such as DMF and NMP, the C12-derivative is only soluble in solvents with lower polarity, such as chloroform and tetrahydrofuran. Characteristic absorptions of the phthalocyanine ring were observed at 362, 635 and 693 nm in the UV-visible spectrum of 2 in CHCl3. Thin layer chromatography (TLC) on silica with CHCl3 as the eluent indicated that 2 (MW = 1525.42) consists mostly of a single component, with Rf = ∼0.6. The base peak in the negative mode ESI-MS of 2 (Fig. 2) is consistent with the singly-charged stacked dimer 22− (m/z = 3050, Figure S5†), while the next most abundant peak represents the monomeric 2− (m/z = 1525, Figure S6†). Minor peaks corresponding to a chloroform adduct of the stacked dimer, {22+CHCl3}− (m/z = 3169), as well as peaks of stacked oligomers, such as 232− (m/z = 2288), 253− (m/z = 2542), 273− (m/z = 3559) and 252− (m/z = 3813), were also identified. The peak at m/z = 3050 most likely contains contributions from 242− and 263−, in addition to 22−, which all have the same m/z value. Indeed, slight differences between the relative intensities of the peaks of the predicted and observed isotopic patterns can be observed (Figure S6†).
 | ||
| Fig. 2 Negative mode ESI mass spectrum of 2, showing peaks assignable to the C12-functionalized tetraimide. | ||
 | ||
| Scheme 2 Scheme of the transformation of 1a into 2 (R = phthalocyanine skeleton, DMA = N,N-dimethylacetamide). | ||
Due to the strong tendency of phthalocyanines to aggregate in solution as a result of their large, planar structures,12 crystal growing attempts failed to provide single crystals of 1a or 1b suitable for X-ray diffraction. Likewise, extensive aggregation renders even the NMR spectra of their metal-free or diamagnetic metal (zinc) analogues uninterpretable. In the absence of direct structural evidence, elemental analysis combined with titrimetric analysis was initially used in an attempt to assign a structure to the phthalocyanine obtained from PMDA. Oligomers with up to five fused Pc units,5,13 and later with nine or more Pc units were thus reported.14 To date,7g the fused tetrameric formula (Cu4Pc*)(COOH)16, first considered in 195915 and coined in 1982,5 has been most frequently assumed.3,6–8 Interestingly, the same reaction, with different PMDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) copper ratios, has also been used in the preparation of “pure” (CuPc)(COOH)8, which also lacked direct structural proof.16 Further confusion about monomeric and oligomeric/polymeric phthalocyanines was created in the literature due to several authors referring to the parent copper(II) phthalocyanine (CuPc, derived from phthalic acid) as the “monomer”, and to the phthalocyanine derived from pyromellitic acid (which has been shown here to consist of (CuPc)(COOH)8) as an “oligomer” or “polymer”.
copper ratios, has also been used in the preparation of “pure” (CuPc)(COOH)8, which also lacked direct structural proof.16 Further confusion about monomeric and oligomeric/polymeric phthalocyanines was created in the literature due to several authors referring to the parent copper(II) phthalocyanine (CuPc, derived from phthalic acid) as the “monomer”, and to the phthalocyanine derived from pyromellitic acid (which has been shown here to consist of (CuPc)(COOH)8) as an “oligomer” or “polymer”.
In 2008, Opris et al. reported the preparation and characterization of oligomer-free (MPc)(COOH)8 (M = Cu or Zn) from dipentyl-4,5-dicyanophthalate, which is only capable of yielding a mononuclear phthalocyanine.17 Most importantly, it was shown that (MPc)(COOH)8 displays an extremely high dielectric constant, on par with that of the assumed “sheet-polymer”, (Cu4Pc*)(COOH)16, reported in 1985.11 Unlike (CuPc)(COOC5H11)8, exposure of (MPc)(COOH)8 samples to increasing humidity levels resulted in a spectacular increase in the measured dielectric constant and electrical conductivity, indicating that water plays an essential role in the generation and transport of mobile charge carriers. Indeed, carboxylic acid moieties can ionize in the presence of water, resulting in protons being released into the absorbed water layers. In light of these observations, it was suggested that protonic, rather than electronic conduction (widely assumed to arise from nomadic polarization3,6–8,18), is responsible for the effect of water uptake on the dielectric constant and conductivity. It should be noted that extended phthalocyanine polymers can be obtained at much higher temperatures than the ones reported in the synthesis of the assumed (Cu4Pc*)(COOH)16: the electrical conductivity of (NiPc)(COOH)8 was reported to increase by seven orders of magnitude after heating to 480 °C.19 Recently, a scanning tunneling microscopy (STM) study has clearly demonstrated the formation of 2D phthalocyanine polymer films from 1,2,4,5-tetracyanobenzene (pyromellitonitrile) and iron.20
In conclusion, we have shown that the copper(II) phthalocyanine obtained from pyromellitic dianhydride (PMDA) under the reported conditions consists of the mononuclear (CuPc)(COOH)8, rather than fused oligomeric or polymeric species. The associated high dielectric constant and electrical conductivity of the reported materials derived from PMDA as compared to the unsubstituted parent CuPc can be attributed to the peripheral carboxylic acid moieties and not to the extended conjugation of the previously assumed large sheet oligomers. Apparently, only one of the two imide moieties of the difunctional pyromellitic diimide intermediate formed during the reaction of PMDA with urea leads to a phthalocyanine ring, without forming significant amounts of larger, fused oligomers. Although the mechanism of phthalocyanine ring formation from monofunctional starting materials, such as phthalic anhydride and its derivatives, is well documented,9 similar studies involving pyromellitic acid derivatives are yet to be performed.
We thank Western Michigan University for financial support.
References
- M. M. Al-Amar, K. J. Hamam, G. Mezei, R. Guda, N. M. Hamdan and C. A. Burns, Sol. Energy Mater. Sol. Cells, 2012 Search PubMed , submitted.
- N. Phougat, P. Vasudevan and H. S. Nalwa, Metallophthalocyanines as High-Dielectric Constant Materials, in Handbook of Low and High Dielectric Constant Materials and Their Applications, Volume 1: Materials and Processing, ed. H. S. Nalwa, Academic Press, San Diego, CA, 1999, ch. 8, pp. 329–427 Search PubMed.
- Q. M. Zhang, H. Li, M. Poh, F. Xia, Z.-Y. Cheng, H. Xu and C. Huang, Nature, 2002, 419, 284 CrossRef CAS.
- Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential, and Challenges, ed. Y. Bar-Cohen, 2nd edn, SPIE, Bellingham, WA, 2004 Search PubMed.
- B. N. Achar, G. M. Fohlen and J. A. Parker, J. Polym. Sci. A: Polym. Chem. Ed., 1982, 20, 1785 CAS.
- (a) S. Venkatachalam, T. M. Vijayan and S. Packirisamy, Makromol. Chem. Rapid Commun., 1993, 14, 703 CrossRef CAS; (b) H. Xu, Y. Bai, V. Bharti and Z.-Y. Cheng, J. Appl. Polym. Sci., 2001, 82, 70 CrossRef CAS; (c) C. Huang, Q. M. Zhang, G. deBotton and K. Bhattacharya, Appl. Phys. Lett., 2004, 84, 4391 CrossRef CAS; (d) V. Bobnar, A. Levstik, C. Huang and Q. M. Zhang, Phys. Rev. Lett., 2004, 92, 047604 CrossRef CAS; (e) C. Huang, Q. M. Zhang, J. Y. Li and M. Rabeony, Appl. Phys. Lett., 2005, 87, 182901 CrossRef; (f) S. Saravanan, M. R. Anantharaman and S. Venkatachalam, Mater. Sci. Eng., B, 2006, 135, 113 CrossRef CAS; (g) V. Bobnar, A. Levstik, C. Huang and Q. M. Zhang, Ferroelectrics, 2006, 338, 107 CrossRef CAS; (h) S. Zhang, C. Huang, R. J. Klein, F. Xia, Q. M. Zhang and Z.-Y. Cheng, J. Intell. Mater. Syst. Struct., 2007, 18, 133 CrossRef CAS; (i) J. Zhang, D. Zhu and M. Matsuo, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 1146 CrossRef CAS.
- (a) J.-W. Wang, Q.-D. Shen, C.-Z. Yang and Q.-M. Zhang, Macromolecules, 2004, 37, 2294 CrossRef CAS; (b) J.-W. Wang, Q.-D. Shen, H.-M. Bao, C.-Z. Yang and Q.-M. Zhang, Macromolecules, 2005, 38, 2247 CrossRef CAS; (c) C. Huang and Q. M. Zhang, Adv. Mater., 2005, 17, 1153 CrossRef CAS; (d) Y. Wang, J.-W. Wang, F. Wang, S. Li and J. Xiao, Polym. Bull., 2008, 60, 647 CrossRef CAS; (e) J.-W. Wang, Y. Wang, F. Wang, S.-Q. Li, J. Xiao and Q.-D. Shen, Polymer, 2009, 50, 679 CrossRef CAS; (f) J.-W. Wang, Y. Wang, S.-Q. Li and J. Xiao, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 490 CrossRef CAS; (g) R. Saha and B. K. Mandal, J. Appl. Polym. Sci., 2010, 117, 122 CrossRef CAS.
- Z.-M. Dai, Y. Gao, H.-P. Xu and J. Bai, J. Colloid Interface Sci., 2008, 322, 491 CrossRef.
- The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, 2003, vols. 15–20 Search PubMed.
- K. J. Hamam, M. M. Al-Amar, G. Mezei, R. Guda and C. A. Burns, manuscript in preparation.
- (a) H. S. Nalwa, L. R. Dalton and P. Vasudevan, Eur. Polym. J., 1985, 21, 943 CrossRef CAS; (b) U. S. Tewari, S. K. Sharma, H. S. Nalwa and P. Vasudevan, IEEE Trans. Electr. Insul., 1985, EI–20, 975 CrossRef CAS.
- (a) A. Suchan, J. Nackiewicz, J. Hurek and W. Waclawek, Polish J. Chem., 2005, 79, 1937 CAS; (b) A. Suchan and J. Nackiewicz, Polish J. Chem., 2008, 82, 1489 CAS.
- D. R. Boston and J. C. Bailar Jr., Inorg. Chem., 1972, 11, 1578 CrossRef CAS.
- D. Wöhrle and E. Preußner, Makromol. Chem., 1985, 186, 2189 CrossRef.
- W. C. Drinkard and J. C. Bailar Jr., J. Am. Chem. Soc., 1959, 81, 4795 CrossRef CAS.
- (a) H. Shirai, A. Maruyuma, J. Takano, K. Kobayashi and N. Hojo, Makromol. Chem., 1980, 181, 565 CrossRef CAS; (b) T. Sekine, K. Kimura and K. Yoshihara, J. Nucl. Sci. Technol., 1986, 23, 1064 CrossRef CAS; (c) K. Ogawa, S. Kinoshita, H. Yonehera, H. Nakahara and K. Fukuda, J. Chem. Soc., Chem. Commun., 1989, 477 RSC; (d) E. J. Osburn, L.-K. Chau, S.-Y. Chen, N. Collins, D. F. O'Brien and N. R. Armstrong, Langmuir, 1996, 12, 4784 CrossRef CAS; (e) K. Sakamoto and E. Ohno, Prog. Org. Coat., 1997, 31, 139 CrossRef CAS; (f) D. Manno, R. Rella, A. Serra, P. Siciliano, A. Taurino, L. Troisi and L. Valli, Mater. Sci. Eng., C, 1998, 5, 317 CrossRef; (g) B. Wang and H.-Z. Ma, Synth. React. Inorg. Met.-Org. Chem., 2004, 34, 1009 CrossRef CAS.
- D. M. Opris, F. Nüesch, C. Löwe, M. Molberg and M. Nagel, Chem. Mater., 2008, 20, 6889 CrossRef CAS.
- H. A. Pohl, IEEE Trans. Electr. Insul., 1986, EI–21, 683 CrossRef CAS.
- S. Venkatachalam, K. V. C. Rao and P. T. Manoharan, Synth. Met., 1988, 26, 237 CrossRef CAS.
- M. Abel, S. Clair, O. Ourdjini, M. Mossoyan and L. Porte, J. Am. Chem. Soc., 2011, 133, 1203 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: a literature survey of the phthalocyanines obtained from the reaction of pyromellitic acid derivatives with urea and metal salts, and details of the mass spectrometric analysis. See DOI: 10.1039/c2ra21634e |
| ‡ 500 mg of 1a was refluxed in 10 ml acetic anhydride for one day under stirring, followed by filtration, rinsing with chloroform and drying in a vacuum. The resulting solid was added to a solution of 2.61 g 1-dodecylamine in 50 ml N,N-dimethylacetamide (DMA), and was refluxed for two days under stirring. The product (2) was filtered out and washed with DMA and methanol, and was dried in a vacuum (yield: 550 mg). |
| This journal is © The Royal Society of Chemistry 2012 |
