Facile synthesis of layered LiV3O8 hollow nanospheres as superior cathode materials for high-rate Li-ion batteries†
Jun
Liu
*,
Wei
Liu
,
Yanling
Wan
,
Shaomin
Ji
*,
Jinbin
Wang
and
Yichun
Zhou
*
Key Laboratory of Low Dimensional Materials & Application Technology, Ministry of Education, Faculty of Materials, Optoelectronics and Physics, Xiangtan University, 411105, China. E-mail: jliu@xtu.edu.cn; smji@xtu.edu.cn; zhouyc@xtu.edu.cn; Fax: (+) 86-731-58293586; Tel: (+)86-731-58298119
First published on 4th September 2012
Abstract
Layered LiV3O8 porous and hollow nanospheres were synthesized via a facile solid-phase oxidization and lithiation route with V2O3 hollow nanospheres as the precursor. When used as the cathode for Li-ion batteries, the as-obtained LiV3O8 porous and hollow nanospheres showed a high specific capacity of 324 mA h g−1 at a current density of 0.1 C, stable capacity (250 mA h g−1 after 50 cycles), and excellent rate capability (124 mA h g−1 at 5 C).
Exploring cathode material with larger discharge specific capacity is still the main challenge for developing Li-ion batteries because their electrochemical performances are usually cathode limited.1–5 Cathode materials are typically lithium oxides of transition metals, which can undergo oxidation to higher valences when lithium is removed. While oxidation of the transition metal can retain charge neutrality in the compound, large compositional changes often lead to phase changes, so crystalline structures that are stable over wide ranges of composition must be used.6,7 Presently, there are three intercalation materials applied commercially as cathode materials for rechargeable Li-ion batteries: LiCoO2, LiNiO2, and LiMn2O4.6,8 Another promising class of cathode materials are phosphates (LiMPO4, M = Fe, Mn, Co) with the olivine structure (Pnma), in which phosphorous occupies tetrahedral sites, the transition metal (M) occupies octahedral sites and lithium forms one-dimensional chains along the [010] direction.9–14 The phosphate most commonly used for the cathode is LiFePO4, which delithiates to FePO4 as the Fe2+ is oxidized to Fe3+.15 Cathode materials have achieved a great success in practical application, but alternatives have are being developed which have lower cost, improved stability, and higher specific capacity. Vanadium oxide forms layered compounds and vanadium can have multiple valences, so vanadium oxides can be used as electrode materials for Li-ion batteries.16 Vanadium oxide-based cathode materials have been attracting much attention due to their low-cost, high discharge capacity and high specific energy.17–24
Among them, the layered lithium trivanadate (LiV3O8) can insert reversibly over three Li per formula unit at the tetrahedral sites, making it one of the most promising substitutes for expensive LiCoO2 cathodes used commercially in Li-ion batteries.21,25,26 However, the LiV3O8 synthesized by traditional solid-state reaction route usually suffers from serious capacity loss caused by poor kinetic diffusion of Li ions into LiV3O8 tetrahedral sites.25,26 To overcome this obstacle, much research has been focused on improving the electrochemical performances of LiV3O8 by either developing new synthetic routes or structural/morphological modifications.27–32 Previous research has shown that the particle morphology and crystal shape play a major role on the initial capacity and cyclability of LiV3O8 cathode.27–29 Reducing the electrode particle size and increasing their surface area can improve Li ion diffusion kinetics, leading to higher specific capacity and better cyclability. For example, Wang and co-workers have demonstrated that LiV3O8 nanorods exhibit a high discharge capacity of 302 mA h g−1 and remain at 278 mA h g−1 after 30 cycles.27 Zhou et al. have shown that LiV3O8 single-crystalline nanorods exhibit an initial discharge capacity of 348 mA h g−1 at 20 mA g−1, and still remain at 303 mA h g−1 at 50 mA g−1.29 Recently, Liang and co-workers have demonstrated that the LiV3O8 nanorods prepared by a thermal co-decomposition method deliver specific discharge capacities of 320 mA h g−1 and 239 mA h g−1 at current densities of 100 mA g−1 and 1 A g−1, respectively, and also exhibit good capacity retention.30 Especially, hollow-structured electrodes such as hollow micro/nanospheres and nanotubes, are special and unique. Besides their large surface area and short effective diffusion distance for Li ions, the cavities in hollow nanostructured electrodes may provide extra space for the storage of Li-ions, beneficial for enhancing specific capacity.33,34
The traditional method for preparation of LiV3O8 cathode materials is to employ bulk V2O5 or NH4VO3 as the vanadium precursor, accomplished by solid-state reaction.25,26 However, such procedures commonly give the LiV3O8 as bulk material. Though great achievements have been made in improving the specific capacity and cycle stability of LiV3O8 cathode via the above structural/morphological modifications,27–32 its electrochemical performances still need further enhancement for the practical application in electric vehicles and hybrid electric vehicles. To the best of our knowledge, the controllable synthesis and electrochemical properties of layered LiV3O8 with porous and hollow nanosphere structure have not been reported yet due to the difficulty in preparing such hollow nanostructures by a facile method. Previously, we have successfully constructed hierarchical double-shelled nanocapsules assembled from the V2O5 matrix and SnO2 nanoparticles, which show superior electrochemical performances when used as electrode materials for Li-ion batteries.22 Lately, by utilizing the crystallization process of Ostwald ripening, we reported the synthesis of yolk-shell V2O5 spheres from V2O3 spheres as cathode materials for Li-ion batteries.23 Through rationally tuning the parameters of this reaction system, completely hollow-structured vanadium oxide spheres can be achieved. Herein, we describe the use of these home-made V2O3 hollow nanospheres for the first time as vanadium precursor combined with LiOH, novel LiV3O8 porous and hollow nanospheres were fabricated and characterized. When used as cathode materials in rechargeable Li-ion batteries, the as-prepared LiV3O8 porous and hollow nanospheres exhibited high discharge capacity, superior rate capacity and stable cycling performance.
The LiV3O8 porous and hollow nanospheres were achieved using V2O3 hollow nanospheres obtained from a controlled Ostwald ripening process as templates, which is illustrated in Scheme 1a. First, these V2O3 hollow nanosphere precursors were controllably obtained by a solvothermal crystallization route through an inward Ostwald ripening process (Fig. S1, ESI†).23 Following this, these newly-formed low-valence V2O3 hollow nanosphere precursors with high activity were oxidized and lithiated completely into well-crystallized LiV3O8 hollow nanospheres via calcination with LiOH in ambient air. During this solid-phase transformation process, owning to their small size and high activity, these newly-formed V2O3 hollow nanospheres were easily converted into lithiated vanadium oxide LiV3O8 with layered structure (Scheme 1b). The current synthesis method is simple and easily scaled up (see ESI† for details).
 | ||
| Scheme 1 (a) Schematic mechanism for the fabrication process of hollow-structured LiV3O8 nanospheres based on Ostwald ripening and solid transformation processes of V2O3 hollow nanospheres. (b) Solid-phase transformation of rhombohedral V2O3 into layered monoclinic LiV3O8via calcination with LiOH in ambient air. | ||
The as-prepared V2O3 precursor was firstly characterized as shown in Fig. 1. Fig. 1a displays a panoramic scanning electron microscope (SEM) image of the V2O3 hollow nanospheres. As seen from the image, the hollow nanospheres have a regular shape with uniform diameter of about 300–500 nm. The nanosphere surface is relatively smooth, suggesting a complete crystallization process of the precursor under the present solvothermal condition (Fig. 1b,c). Transmission electron microscopy (TEM) image of these precursor nanospheres shown in the inset of Fig. 1c strongly confirms that these nanospheres have completely hollow cores. X-ray diffractometry (XRD) patterns of these V2O3 nanosphere precursors are given in Fig. 1d. All the peaks can be well-indexed to rhombohedral V2O3 phase (JCPDS card No. 34-0187). As shown in the inset of Fig. 1d, rhombohedral V2O3 shows the corundum structure, in which the vanadium atoms form a honeycomb lattice in the ab plane and are arranged in V–V pairs along the hexagonal c axis derived from an ideal chain structure by introducing vacancies at every third site, and the oxygen atoms form distorted octahedral structures around the vanadium sites. The octahedra are interlinked via facets along the c axis and edges and corners perpendicular to this axis, as the V–V pair along the hexagonal c axis shares octahedral faces.35
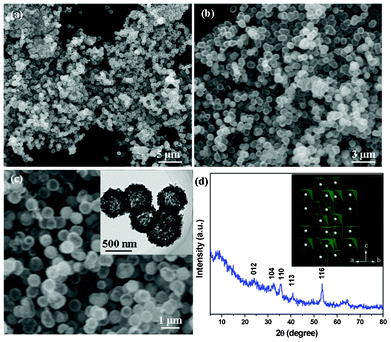 | ||
| Fig. 1 Different magnification SEM images (a–c) and XRD patterns (d) of hollow-structured V2O3 nanosphere precursors. The inset of Fig. 1c displays a TEM image of hollow-structured V2O3 nanosphere precursors, and the inset of Fig. 1d shows a crystalline structure of rhombohedral V2O3 precursors. | ||
Fig. 2a shows a panoramic SEM image of the finally achieved LiV3O8 hollow nanospheres. As can be seen from the image, the LiV3O8 products almost retain the precursor's spherical shape with 300–500 nm in diameter. While comparing Fig. 2a with Fig. 1a, it is apparent the LiV3O8 nanospheres are somewhat more irregular in shape and rougher in surface than those of V2O3 nanosphere precursors. This microstructure transformation can be ascribed to the rearrangement of the atoms due to the crystalline structure and lattice parameters which have changed during the lithiation and oxidization of V2O3 nanospheres. Higher magnification SEM images of these LiV3O8 nanospheres are displayed in Fig. 2b and c, which clearly show the outer rough surface of these hollow nanospheres. The crystalline phase of these LiV3O8 nanospheres were characterized by XRD and are shown in Fig. 2d. All the diffraction peaks can be indexed to pure monoclinic structure phase of space group p21/m LiV3O8 with the unit cell parameters of a = 6.680, b = 3.596, c = 12.024 Å, which is in agreement with the layered LiV3O8 (JCPDS card No. 72-1193). The sharp and intense XRD peaks of the achieved LiV3O8 nanospheres reveal their well-crystallization.
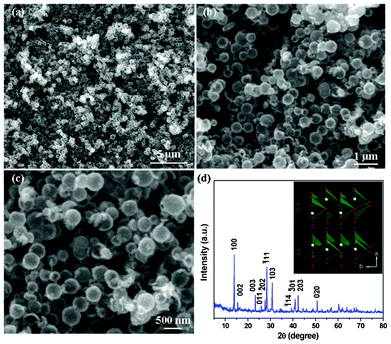 | ||
| Fig. 2 Different magnification SEM images (a–c) and XRD patterns (d) of hollow-structured LiV3O8 nanosphere products. The inset of Fig. 2d shows crystalline structure of layered monoclinic LiV3O8 products. | ||
All these morphology and structure details of the LiV3O8 porous and hollow nanospheres are further characterized by TEM, as shown in Fig. 3a–c. Fig. 3a shows a typical low-magnification image of LiV3O8 hollow nanospheres, which indicates that the product is composed of hollow nanospheres with uniform size. It was observed that the contrast between the central portion and the edge of nanospheres strongly supports the formation of hollow nanostructures. A higher magnification TEM image (Fig. 3b) reveals that the porous shell is a hierarchical nanostructure composed of dozens of tiny LiV3O8 nanoparticles with a size of several tens of nanometers. The hollow nature of the as-prepared porous nanospheres can be further proven by the high-magnification TEM image of a single nanosphere (Fig. 3c). The characteristics of polycrystalline porous nanostructures can be confirmed by the selected-area electron diffraction (SAED) pattern (the inset of Fig. 3d) of a single nanosphere as shown in Fig. 3c. Lattice plane with the interplanar distance of 0.310 nm was observed in the lattice fringe (Fig. 3d), which corresponds to the (111) plane of the LiV3O8 monoclinic crystalline structure. The surface area of these LiV3O8 porous and hollow nanospheres were measured using the Brunauer-Emmett-Teller (BET) method. As shown in Fig. S2 (ESI†), the N2 adsorption–desorption isotherm at 77 K can be classified as a typical III isotherm, and has two hysteresis loops in the relative pressure range of 0.4–1, indicating bimodal pore-size distributions in the mesoporous and macroporous region (the inset in Fig. S2, ESI†). The first hysteresis loop is at a low relative pressure (0.4 < P/P0 < 0.8), corresponding to the filling of the framework being confined to smaller mesopores formed between intra-agglomerated primary nanoparticles. The second hysteresis loop is at a high relative pressure (0.83 < P/P0 < 1), corresponding to the filling of larger textural mesopores produced by inter-aggregated secondary particles. This bimodal pore-size distribution is further confirmed by its corresponding pore-size distribution curve (the inset in Fig. S2, ESI†) calculated from the desorption branch of the nitrogen isotherm by the BJH method. The powder contains mesopores with small mesopores (4.10 nm) and large mesopores with a maximum pore diameter of ca. 30 nm (a BET specific surface area of about 92.56 m2 g−1). It has been well-documented that these porous and hollow nanostructured materials may facilitate the electrochemical insertion/extraction of Li ions among the host material, and the highly porous surface may provide more active sites, therefore, improved electrochemical performances of the present LiV3O8 hollow nanospheres would be expected.
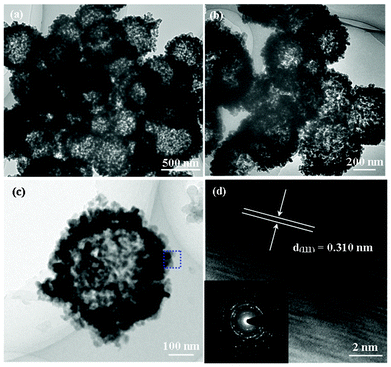 | ||
| Fig. 3 TEM characterizations of hollow-structured LiV3O8 nanosphere cathodes: (a) low-magnification TEM image; (b) higher magnification TEM image; (c) high-magnification TEM image of a single LiV3O8 hollow nanosphere; (d) HRTEM image of the selected area in 3c, revealing lattice planes of LiV3O8 hollow nanosphere, and the bottom inset exhibits SAED pattern of a single nanosphere, which indicates that these LiV3O8 hollow nanospheres are polycrystalline. | ||
Layered LiV3O8 cathode, firstly reported by Wadsley and co-workers,36 is composed of two basic structure units (VO6 octahedron and VO5 distorted trigonal bipyramid) and the V3O8− layers are held together through interaction with the interlayered Li ions (inset of Fig. 2d). It shows much better structure stability than other vanadates,30,31 such as V2O5 cathode materials, making it a very promising cathode material for high power Li-ion batteries. As the current LiV3O8 materials have a porous shell and hollow core, it allows Li ions to intercalate easily, leading to the improvement of the Li intercalation performances. The galvanostatic first 25 cycles of charge/discharge behaviors of the LiV3O8 hollow nanospheres recorded over the potential range between 2.0 and 4.0 V vs. Li/Li+ at a current density of 0.1 C (10 h per half cycle, 30 mA g−1) are shown in Fig. 4a. Theoretically, LiV3O8 can insert four Li per formula unit (corresponding to a high theoretical specific capacity of about 381 mA h g−1), which induces several phase transformations between couples of Li1+xV3O8 (x = 0.1 − 4).37,38 The discharge capacity of the prepared LiV3O8 hollow nanospheres is about 324 mA h g−1, corresponding to about 3.4 Li being intercalated to the LiV3O8 materials, and it still delivers a capacity of 251 mA h g−1 (about 2.7 Li) after 25 cycles at 0.1 C rate. The first discharge capacity of 324 mA h g−1 is little smaller than the theoretical specific capacity of LiV3O8 cathode, which may be ascribed to the low electric conductivity and poor kinetic diffusion of Li ions of bare LiV3O8 cathode material,25,26 and the following work can be carried out by combining with conductive material coating or efficient element doping. Fig. 4b shows the cyclic behavior and Coulombic efficiency of the hollow LiV3O8 cathode at 0.1 C rate. After 50 cycles, the LiV3O8 hollow nanospheres can still deliver a reversible capacity of 250 mA h g−1, which reaches 77.2% of its initial capacity, whereas the Coulombic efficiency steadily kept the values higher than 90% except the 21st cycle.
 | ||
| Fig. 4 Electrochemical performances of LiV3O8 cathode materials: (a) voltage-capacity curves of LiV3O8 hollow nanospheres in the first 25 discharge/charge cycles measured in the voltage range of 2.0–4.0 V at 0.1 C rate; (b) cycling performance of LiV3O8 hollow nanosphere at 0.1 C rate; (c) discharge/charge curves of LiV3O8 hollow nanospheres at different rates; (d) cycling performance of LiV3O8 hollow nanospheres at different rates. | ||
Charge/discharge curves at different current densities (Fig. 4c) show that these LiV3O8 hollow nanospheres exhibit a good rate capability as the cathode materials. Even at a high current density of 5 C, this material can still deliver a reversible capacity of 124 mA h g−1. Fig. 4d demonstrates the superior rate capability of LiV3O8 hollow nanospheres, with the discharge capacity of 243, 222, 185 mA h g−1 at 0.4, 0.8, 1 C, respectively. Even at the rates of 2 and 5 C, it still retains a high value of 153 and 124 mA h g−1, respectively. When the current density was further increased into a higher current density of 20 C, these LiV3O8 porous and hollow nanospheres can still deliver a reversible capacity of about 80 mA h g−1 (Fig. S3, ESI†). The electrochemical performance of these LiV3O8 hollow nanospheres is much better compared to that of V2O5 cathode materials, such as our previously prepared double-shelled V2O5-based nanocapsules (174 mA h g−1 after 50 cycles at 100 mA g−1)22 and V2O5 yolk-shell spheres (220 mA h g−1 after 30 cycles at 60 mA g−1).23 The improved cycle stability of LiV3O8 cathode compared to that of V2O5 cathode is ascribed to its higher structure stability during Li ions insertion/extraction. The electrochemical performance of these LiV3O8 porous and hollow nanospheres is also better compared to that of solid LiV3O8 nanospheres and LiV3O8 bulk materials obtained by conventional solid-state reaction employing bulk V2O5 as the vanadium precursor (Fig. S4, S5, ESI†). When compared with other nanostructured LiV3O8 cathode materials, such as LiV3O8 nanorods27 and nanosheets,31 our produced LiV3O8 porous and hollow nanospheres still show higher specific capacity and rate capability. The excellent electrochemical performance of these LiV3O8 hollow nanospheres is believed to be the result of their unique porous and hollow nanosphere morphology. Several probable reasons could be proposed. Firstly, as mentioned before, the large pores among nanoparticles can be easily filled with the electrolyte, ensuring a highly porous surface is in contact with the electrolyte, and hence a large flux of Li ions across the interface.11,21 Secondly, the diameter of nanospheres and size of tiny nanoparticles constructed nanospheres are both small, rendering a very short transport length for Li ions during insertion/extraction.30,31,39,40 More importantly, the cavity among individual nanoparticle subunits (in porous shell) and hollow cores serve as a good cushion for the material volume changes during Li ions insertion/extraction, which can well enhance cycling performances.33,34,41
In summary, we have employed home-made V2O3 hollow nanospheres as the vanadium precursor to prepare the layered LiV3O8 cathode materials. The as-obtained polycrystalline LiV3O8 porous and hollow nanospheres with high crystallinity greatly improved the stability of the crystallographic structure during cycling. This nanostructured LiV3O8 cathode material exhibited a high discharge capacity of 324 mA h g−1 recorded the current density of 0.1 C; and still delivered 250 mA h g−1 after 50 cycles. Moreover, it also displays an excellent cyclability when cycled at the range of 0.4 C to 5 C. Results reported here support LiV3O8 porous and hollow nanospheres as promising cathode materials for high rate Li-ion battery applications.
Acknowledgements
This work was financially supported by the China Postdoctoral Science Foundation (2011M501279, 2012T50654), Scientific Research Fund of Hunan Provincial Education Department (11C1198), and the National Natural Science Foundation of China (11202177, 51202207).References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- F. Lu, Y. C. Zhou, J. Liu and Y. Pan, Electrachim. Acta, 2011, 56, 8833 CrossRef CAS.
- K. Saravanan, P. Balaya, M. V. Reddy, B. V. R. Chowdari and J. J. Vittal, Energy Environ. Sci., 2010, 3, 457 CAS.
- J. Liu, T. E. Conry, X. Y. Song, M. M. Doeff and T. J. Richardson, Energy Environ. Sci., 2011, 4, 885 CAS.
- Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251 CrossRef CAS.
- H. Song, K. T. Lee, M. G. Kim, L. F. Nazar and J. Cho, Adv. Mater., 20, 3818 CAS.
- C. X. Guo, M. Wang, T. Chen, X. W. Lou and C. M. Li, Adv. Energy Mater., 2011, 1, 736 CrossRef CAS.
- C. X. Guo, Y. Q. Shen, Z. L. Dong, X. D. Chen, W. W. Lou and C. M. Li, Energy Environ. Sci., 2012, 5, 6919 CAS.
- W. He, J. F. Qian, Y. L. Cao, X. P. Ai and H. X. Yang, RSC Adv., 2012, 2, 3423 RSC.
- R. Malik, F. Zhou and G. Ceder, Nat. Mater., 2011, 10, 587 CrossRef CAS.
- Y. K. Sun, S. T. Myung, B. C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320 CrossRef CAS.
- P. Gibot, M. Casas-Cabanas, L. Laffont, S. Levasseur, P. Carlach, S. Hamelet, J. M. Tarascon and C. Masquelier, Nat. Mater., 2008, 7, 741 CrossRef CAS.
- P. Barpanda, M. Ati, B. C. Melot, G. Rousse, J. N. Chotard, M. L. Doublet, M. T. Sougrati, S. A. Corr, J. C. Jumas and J. M. Tarascon, Nat. Mater., 2011, 10, 772 CrossRef CAS.
- N. Meethong, Y. H. Kao, W. C. Carter and Y. M. Chiang, Chem. Mater., 2010, 22, 1088 CrossRef CAS.
- X. H. Rui, J. X. Zhu, W. L. Liu, H. T. Tan, D. H. Sim, C. Xu, H. Zhang, J. Ma, H. H. Hng, T. M. Lim and Q. Y. Yan, RSC Adv., 2011, 1, 117 RSC.
- A. Sakunthala, M. V. Reddy, S. Selvasekarapandian, B. V. R. Chowdari and P. C. Selvin, Energy Environ. Sci., 2011, 4, 1712 CAS.
- D. Yu, C. Chen, S. Xie, Y. Liu, K. Park, X. Zhou, Q. Zhang, J. Li and G. Cao, Energy Environ. Sci., 2011, 4, 858 CAS.
- V. Pralong, V. Gopal, V. Caignaert, V. Duffort and B. Raveau, Chem. Mater., 2012, 24, 12 CrossRef CAS.
- A. Manthiram and J. Kim, Chem. Mater., 1998, 10, 2895 CrossRef CAS.
- N. A. Chemova, M. Roppolo, A. C. Dillon and M. S. Whittingham, J. Mater. Chem., 2009, 19, 2526 RSC.
- J. Liu, H. Xia, D. Xue and L. Lu, J. Am. Chem. Soc., 2009, 131, 12086 CrossRef CAS.
- J. Liu, Y. C. Zhou, J. B. Wang, Y. Pan and D. Xue, Chem. Commun., 2011, 47, 10380 RSC.
- S. Wang, S. Li, Y. Sun, X. Feng and C. Chen, Energy Environ. Sci., 2011, 4, 2854 CAS.
- N. Dupre, J. Gaubicher, D. Guyomard and C. P. Grey, Chem. Mater., 2004, 16, 2725 CrossRef CAS.
- P. Rozier, M. Morcrette, P. Martin, L. Laffont and J. M. Tarascon, Chem. Mater., 2005, 17, 984 CrossRef CAS.
- H. Y. Xu, H. Wang, Z. Q. Song, Y. W. Wang, H. Yan and M. Yoshimura, Electrachim. Acta, 2004, 49, 349 CrossRef CAS.
- Y. Gu, D. Chen, X. Jiao and F. Liu, J. Mater. Chem., 2006, 16, 4361 RSC.
- H. Liu, Y. Wang, K. Wang, Y. Wang and H. Zhou, J. Power Sources, 2009, 192, 668 CrossRef CAS.
- A. Pan, J. Liu, J. G. Zhang, G. Cao, W. Xu, Z. Nie, X. Jie, D. Choi, B. W. Arey, C. Wang and S. Liang, J. Mater. Chem., 2011, 21, 1153 RSC.
- A. Pan, J. G. Zhang, G. Cao, S. Liang, C. Wang, Z. Nie, B. W. Arey, W. Xu, D. Liu, J. Xiao, G. Li and J. Liu, J. Mater. Chem., 2011, 21, 10077 RSC.
- H. Wang, Y. Ren, Y. Wang, W. Wang and S. Liu, CrystEngComm, 2012, 14, 2831 RSC.
- J. Liu, H. Xia, L. Lu and D. Xue, J. Mater. Chem., 2010, 20, 1506 RSC.
- J. Liu and D. Xue, Nanoscale Res. Lett., 2010, 5, 1619 CrossRef CAS.
- P. D. Dernier, J. Phys. Chem. Solids, 1970, 31, 2569 CrossRef CAS.
- A. D. Wadsley, Acta Crystallogr., 1957, 10, 261 CrossRef CAS.
- S. Jouanneau, A. Salle, A. Verbaere, M. Deschamps, S. Lascaud and D. Guyomard, J. Mater. Chem., 2003, 13, 921 RSC.
- S. Jouanneau, A. Salle, A. Verbaere and D. Guyomard, J. Electrochem. Soc., 2005, 152, A1660 CrossRef CAS.
- J. Liu, Y. C. Zhou, J. B. Wang, Y. Pan and D. Xue, CrystEngComm, 2012, 14, 2669 RSC.
- J. Liu, Y. C. Zhou, J. B. Wang, Y. Pan and D. Xue, RSC Adv., 2012, 2, 2262 RSC.
- J. Liu, Y. L. Wan, C. P. Liu, W. Liu, S. M. Ji, Y. C. Zhou and J. B. Wan, Enr. J. Inorg. Chem., 2012, 24, 3825 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c2ra20969a |
| This journal is © The Royal Society of Chemistry 2012 |
