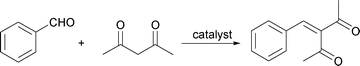
Knoevenagel condensations of 1,3-dicarbonyl compounds with aldehydes catalyzed by heterogeneous Ps-AlCl3 without solvents†
Yi
Zhang
,
Qianqian
Dou
,
Liyan
Dai
*,
Xiaozhong
Wang
and
Yingqi
Chen
Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, P. R. China. E-mail: dailiyan@zju.edu.cn; Fax: +86-571-87952693; Tel: +86-571-87952693
First published on 26th July 2012
Abstract
A simple, efficient procedure is proposed for Knoevenagel condensations of 1,3-dicarbonyl compounds with aldehydes catalyzed by heterogeneous polystyrene-supported aluminum chloride (Ps-AlCl3) under solvent-free conditions. The condensations are carried out smoothly with high yields (87–98%) at 60 °C for 2–4 h in the presence of the Ps-AlCl3 catalyst. The catalyst is characterized by Fourier transfer-infrared spectroscopy (FT-IR). The catalyst is applicable to a wide range of aldehydes, and has excellent recyclability and can be reused several times without loss of activity.
Introduction
Knoevenagel condensation is a well-known reaction for carbon–carbon bond formation in organic synthesis. The adducts such as α,β-unsaturated carbonyl compounds and α,β-unsaturated esters have been used as intermediates in cosmetics and perfumes, natural products, functional polymers, and pharmaceutical applications.1–3 Due to its wide applications, many homogeneous catalyst systems4–11 have been developed for this reaction. Because of the difficulty in separation of catalysts from products and/or solvents and high Chemical Oxygen Demand (COD) in wastewater generated, industrial utilization of those catalysts on a large scale is still unfavorable.In recent years, the growing public concerns about environmental pollution have caused the chemical industry to minimize wastes generated during chemical manufacturing. Heterogeneous catalysis is a more clean and environmentally-friendly catalytic method. Many heterogeneous catalysts such as basic MCM-41,12,13 amine-functionalized materials,14–17 montmorillonite KSF,1 cationic coordination cage,18 zeolites,19,20 enzymes,21,22 and ionic liquids23–26 have been developed to catalyze Knoevenagel condensations. These heterogeneous catalysts have higher atom efficiency, stability, operational simplicity, selectivity and recyclability.
With the development of science and the progress of human society, there is an increasing demand to minimize the usage of toxic volatile solvents and use water as an alternative solvent in chemical reactions. Although numerous clean procedures for Knoevenagel reactions have been practised in water or under solvent-free conditions, most of the condensations are reactions of nitrile compounds with aldehydes.27,28 The existing green technologies are not well suited for the condensations of 1,3-dicarbonyl compounds since these compounds have an inherent tendency to form a stable six-membered enolate which makes it less reactive.29,30 Only a few groups have undertaken trials, with insignificant progress.19,31,32 Therefore, it is desirable to develop and characterize highly efficient and reusable heterogeneous catalysts for Knoevenagel condensations between aldehydes and 1,3-dicarbonyl compounds either without solvents or in water.
In this paper, we report in detail a study using Ps-AlCl333–35 as an efficient heterogeneous catalyst for Knoevenagel condensations of 1,3-dicarbonyl compounds (Scheme 1) with various aromatic aldehydes under solvent-free and mild conditions. The experimental results indicate high yields. The water stable Ps-AlCl3 catalyst can be easily recycled from the reaction system using simple filtration and can be reused several times without loss of activity, which provides a green route for Knoevenagel reactions.
 | ||
| Scheme 1 Knoevenagel condensations of 1,3-dicarbonyl compounds with aldehydes catalyzed by Ps-AlCl3. | ||
Results and discussion
Characterization
Polystyrene-supported aluminum chloride (Ps-AlCl3) was prepared by immobilizing anhydrous aluminum chloride on polystyrene (8% divinylbenzene) according to a procedure reported in the literature.36 Although free AlCl3 is hygroscopic and corrosive, polystyrene supported AlCl3 is nonhygroscopic, stable, and can be stored in air for a few months without significant loss of activity. Ps-AlCl3 was characterized by FT-IR and pyridine-adsorbed FT-IR.The infrared spectra of polystyrene and Ps-AlCl3 are shown in Fig. 1. The IR spectrum of polystyrene is nearly the same before and after the immobilization of AlCl3. The successful immobilization of AlCl3 onto Ps was demonstrated by a new band at 1637 cm−1 in the IR spectrum of Ps-AlCl3.36
 | ||
| Fig. 1 FT-IR spectra of Ps and Ps-AlCl3. | ||
The IR spectra of pyridine adsorbed on two Ps-Lewis acids samples in Fig. 2 shows the characteristic band at 1450 cm−1, which was attributed to Lewis acid sites,37 but no band at the same wavelength was observed for the Ps sample. Again this proved that the Lewis acids were successfully immobilized on polystyrene.
Catalytic studies
Generally, liquid-phase Knoevenagel condensations are carried out in the presence of weak bases or Lewis acids. For a variety of Lewis acids, TiCl4 is a powerful and effective catalyst for the condensations,4,38 but it is water unstable and reacts with water severely, therefore strictly anhydrous conditions and stoichiometric amounts or more are required in order to achieve good yields. Moreover, several molar equivalents of triethylamine to titanium tetrachloride are also needed to improve the reaction yields. To overcome these drawbacks, we proposed using aluminum chloride instead of titanium tetrachloride as the catalyst for the condensation of benzaldehyde with acetylacetone. Experiments indicated that 22 mol% AlCl3 was sufficient for the condensation with 100% conversion at room temperature (Table 1).|
|
||||
|---|---|---|---|---|
| Entry | Catalyst | T (°C) | Time (h) | Conversion (%)c |
| a Reaction conditions for free AlCl3: benzaldehyde (4 mmol), acetylacetone (4.5 mmol). b Reaction conditions for Ps-AlCl3: benzaldehyde (1.5 mmol), acetylacetone (10 mmol). c Conversion determined by GC. d Ps-AlCl3 was reused five times. | ||||
| 1 | 100 mol% AlCl3a | rt | 1 | 100 |
| 2 | 50 mol% AlCl3 | rt | 1 | 100 |
| 3 | 22 mol% AlCl3 | rt | 1 | 100 |
| 4 | 15 mol% AlCl3 | rt | 2.5 | 89 |
| 5 | 15 mol% AlCl3 | 40 | 2.5 | 76 |
| 6 | 15 mol% AlCl3 | 50 | 2.5 | 63 |
| 7 | 15 mol% AlCl3 | 60 | 2.5 | 61 |
| 8 | 20 mol% Ps-AlCl3b | 70 | 2.2 | 100 |
| 9 | 20 mol% Ps-AlCl3 | 60 | 2.2 | 100 |
| 10 | 20 mol% Ps-AlCl3 | 50 | 3 | 87 |
| 11 | 20 mol% Ps-AlCl3 | 40 | 3 | 62 |
| 12 | 20 mol% Ps-AlCl3 | rt | 5 | 35 |
| 13 | 15 mol% Ps-AlCl3 | 60 | 2.5 | 100, 100, 98, 92, 83d |
| 14 | 10 mol% Ps-AlCl3 | 60 | 3 | 81 |
| 15 | 5 mol% Ps-AlCl3 | 60 | 4 | 42 |
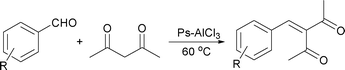
Given the promising results in Table 1, we continued to prepare Ps-AlCl3 and test it as a heterogeneous catalyst for the condensation, which could avoid tedious extra work after completion of the reaction and reduce the amount of acidic wastewater generated. The initial reaction was carried out using 20 mol% Ps-AlCl3 (with respect to the AlCl3 content in Ps-AlCl3) relative to benzaldehyde under solvent-free conditions at room temperature. After 5 h, a sample of the reaction mixture was analyzed by GC. The reaction conversion was 35% at rt, and increased with increasing temperature. The optimal temperature was found to be 60 °C with a conversion of 100%. The reason for requiring a higher temperature was that the acidity of Ps-AlCl3 was weaker than that of anhydrous AlCl3. In addition, mesoporous pores and channels of polystyrene were unfavorable for the molecular mass transfer process. At the same time, the GC-MS of the reaction mixture indicated that no Michael adducts or other byproducts were generated. After completion of the condensation, Ps-AlCl3 was filtered out, excess acetylacetone was recycled under reduced pressure, and the residue was almost pure product as confirmed by 1H NMR spectroscopy. The amount of Ps-AlCl3 was also an important factor for the condensation, 5 mol% Ps-AlCl3 gave a conversion of 42% after 4 h while 15 mol% Ps-AlCl3 was sufficient to give a conversion of 100% after 2.5 h, but the reaction did not occur in the absence of the catalyst or only with polystyrene present. To further confirm that the reaction was not catalyzed by free AlCl3 released from the support, Ps-AlCl3 was stirred in acetylacetone for 1 h and filtered out, then benzaldehyde was added to the filtrate and stirred for 2 h at 60 °C, and no reaction was observed.
The generality of the heterogeneous catalyst was also tested in the above optimized Ps-AlCl3-catalyzed system. We tried various substituted benzaldehydes to condense with acetylacetone and ethyl acetoacetate in the presence of 15 mol% Ps-AlCl3. The results are summarized in Table 2 and Table 3. Most condensations proceeded smoothly to give the corresponding adducts in good to excellent yields. The electronic effects of substrates had some impact on the condensation. Aromatic aldehydes bearing electron-withdrawing groups exhibited higher reactivity than those possessing electron-donating groups. Products of ethyl acetoacetate were E and Z configuration isomers and the assignment of stereochemistry of different isomers was made on the basis of the chemical shifts of CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, which migrated to lower field for the E isomers as confirmed by 1H NMR.39 Furthermore, acid and base sensitive heteroaromatic aldehydes reacted with acetylacetone and ethyl acetoacetate perfectly, and excellent yields were obtained using our method.
C, which migrated to lower field for the E isomers as confirmed by 1H NMR.39 Furthermore, acid and base sensitive heteroaromatic aldehydes reacted with acetylacetone and ethyl acetoacetate perfectly, and excellent yields were obtained using our method.
|
|
|||
|---|---|---|---|
| Entry | Aldehyde | Time (h) | Yield (%)b |
| a Reaction conditions: aldehyde (1.5 mmol), acetylacetone (10 mmol), Ps-AlCl3 (0.5 g, 0.225 mmol AlCl3), 60 °C. b Isolated yield after column chromatography. | |||
| 1 |
|
2.5 | 98 |
| 2 |

|
2.0 | 96 |
| 3 |
|
2.8 | 93 |
| 4 |

|
2.1 | 98 |
| 5 |

|
3.5 | 96 |
| 6 |

|
3.5 | 95 |
| 7 |

|
3.7 | 96 |
| 8 |

|
2.3 | 98 |
| 9 |

|
2.8 | 98 |
| 10 |
|
3.0 | 97 |
|
|
||||
|---|---|---|---|---|
| Entry | Aldehyde | Time (h) | Yield (%)b |
Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ec Ec |
a Reaction conditions: aldehyde (1.5 mmol), ethyl acetoacetate (10 mmol), Ps-AlCl3 (0.5 g, 0.225 mmol AlCl3), 60 °C.
b Isolated yield after column chromatography.
c The Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E geometry was determined by 1H NMR. E geometry was determined by 1H NMR.
|
||||
| 1 |
|
2.5 | 98 | 9.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
| 2 |

|
2.1 | 87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 |

|
3.2 | 90 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 |

|
4.0 | 95 | 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.9 3.9 |
| 5 |

|
4.0 | 96 | 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
| 6 |

|
4.0 | 95 | 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.1 4.1 |
| 7 |

|
2.7 | 97 | 8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.4 9.4 |
| 8 |

|
3.0 | 96 | 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
| 9 |
|
3.4 | 95 | 5.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0 |
Heterogeneous catalysts also have the advantages of easy separation as well as reusability, therefore the recyclability and reusability of Ps-AlCl3 was investigated. The Ps-AlCl3 catalyst was separated by simple filtration after reaction, then washed with plenty of ethyl acetate to remove any physisorbed reagents, dried and used in the next reaction under the same reaction conditions. The results showed that 92% conversion was still achieved at the fourth operation.
Continuing on, we also compared the Ps-AlCl3 catalyst with some homogeneous or heterogeneous catalysts reported in the literature for the Knoevenagel condensation (Table 4). As shown in Table 4, in terms of reaction conditions, yields and costs, etc., Ps-AlCl3 has many advantages over reported catalysts.
| Entry | Catalyst | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Substrate: benzaldehyde and acetylacetone. | |||||
| 1 | Ps-AlCl3 | Neat | 60 | 2 | 98 |
| 2 | Yb(OPf)340 | FBS | 80 | 8 | 80 |
| 3 | Organobismuth complex41 | [Bmin]BF4 | rt | 6 | 95 |
| 4 | Silica sulfuric acid32 | Neat | rt | 10 | 66 |
| 5 | Silica functionalized with amino groups42 | Toluene | rt | — | 35 |
| 6 | Zeolites19 | Neat | 140 | 6 | 32.2 |
| 7 | Magnetic Fe2O3 functionalized with ionic liquids43 | Water | 80 | 9 | 91.1 |
| 8 | Perfluoroalkylated pyridine44 | n-Octane | 80 | 8 | 82 |
| 9 | Piperidine AcOH4 | Benzene | Reflux | 18 | 60 |
| 10 | L-Tryptophan5 | DMSO | rt | 16 | 82 |
| 11 | NbCl545 | Neat | rt | 1 | 85 |
| 12 | Magnesium perchlorate30 | Neat | rt | 70 | 55 |
Encouraged by the results above, we then investigated the reaction mechanism. Based on Fontana and Re’s investigation of TiCl4,38 we hypothesized the mechanism of Ps-AlCl3-catalyzed Knoevenagel condensation (Scheme 2). First, the addition of Ps-AlCl3 to acetylacetone results in the formation of an aluminum enolate anion and a hydrion. Second, the hydrion simultaneously polarizes the aldehyde and aluminum enolate anion attacking the carbonyl carbon atom to form a carbon–carbon bond. Finally, the dehydration takes place on the intermediate, then the dissociation of Ps-AlCl3 resulting in the formation of the Knoevenagel adduct.
 | ||
| Scheme 2 The proposed mechanism of Knoevenagel condensation catalyzed by Ps-AlCl3. | ||
Conclusion
Ps-AlCl3 was an efficient catalyst for Knoevenagel condensation of less active 1,3-dicarbonyl compounds with aldehydes. The condensations were carried out smoothly with high yields under solvent-free and mild reaction conditions in the presence of the Ps-AlCl3 catalyst. After completion of the reaction, Ps-AlCl3 could be easily separated from the reaction system via filtration, showing better recyclability and reusability without loss of activity. We believe that this new methodology has contributions to the environmentally-friendly synthesis of substituted electrophilic alkenes.Experimental
General
All the chemicals used were of analytical grade and were used without further purification. Polystyrene (8% divinylbenzene) was purchased from Nanjing Microspheres Hi-Efficiency Isolation Carrier Co., Ltd. IR spectra were recorded on a Shimadzu FTIR-8400S spectrophotometer. GC profiles were recorded on FuLi 9790 with a SE-54 column (30 m × 0.32 mm × 0.5 μm). GC-MS was recorded on Hewlett-Packard system HP 6890 gas chromatograph. 1H and 13C NMR spectra were recorded in CDCl3 using TMS as an internal standard on a Bruker spectrophotometer at 500 and 125 MHz respectively. High resolution mass spectrum of the new compound was recorded on GCTPremier at 70 eV.Catalyst preparation
0.9 g anhydrous AlCl3 was added to polystyrene (8% divinylbenzene, 3.5 g) in carbon disulfide (25 ml). The mixture was stirred under reflux for 2 h, cooled to room temperature and then cold water was cautiously added to hydrolyze the excess aluminum chloride. The mixture was stirred until the deep orange color disappeared and the catalyst became light yellow, then it was filtered, and the polymer beads were collected, washed with water (250 ml), then with ether, finally it was dried to a constant weight under vacuum. The capacity of Ps-AlCl3 based on its chloride content, which was determined by the Mohr titration method, was 0.45 AlCl3 mmol per g of catalyst.36Catalyst characterization
Polystyrene and Ps-AlCl3 were dehydrated in an infrared (IR) cell at 100 °C for 2 h under vacuum before pyridine adsorption. Pyridine was added to the IR cell at 100 °C. Pyridine adsorption IR spectra of polystyrene and Ps-AlCl3 were measured at room temperature after pyridine desorption at 100 °C for 2 h.Knoevenagel condensation
In a typical experiment, a mixture of aldehyde (1.5 mmol), acetylacetone (10 mmol) and Ps-AlCl3 (0.5 g, 0.225 mmol AlCl3) was stirred at 60 °C for 2.5 h. After completion of the reaction, Ps-AlCl3 was filtered and washed with ethyl acetate (3 × 10 ml). The filtrate was concentrated to give the almost pure crude product, which was purified further by column chromatography (petroleum ether/ethyl acetate) if necessary.Selected spectral data
Acknowledgements
The authors would like to thank Dr Zhengbao Wang for infrared spectra and pyridine-adsorbed IR, Nanjing Microspheres Hi-Efficiency Isolation Carrier Co., Ltd for providing polystyrene. This work was supported by the NSFC (No: 21176213), and Zhejiang Key Innovation Team of Green Pharmaceutical Technology (No: 2010R50043).References
- F. Bigi, L. Chesini, R. Maggi and G. Sartori, J. Org. Chem., 1999, 64, 1033–1035 CrossRef CAS.
- N. F. Yu, J. M. Aramini, M. W. Germann and Z. W. Huang, Tetrahedron Lett., 2000, 41, 6993–6996 CrossRef CAS.
- B. A. Robichaud and K. G. Liu, Tetrahedron Lett., 2011, 52, 6935–6938 CrossRef CAS.
- R. Antonioletti, P. Bovicelli and S. Malancona, Tetrahedron, 2002, 58, 589–596 CrossRef CAS.
- Y. Hu, Y.-H. He and Z. Guan, Catal. Commun., 2010, 11, 656–659 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, A. K. Basak, B. Visali, A. V. Narsaiah and K. Nagaiah, Eur. J. Org. Chem., 2004, 546–551 CrossRef CAS.
- A. Palasz and T. Palasz, Tetrahedron, 2011, 67, 1422–1431 CrossRef CAS.
- P. Goswami and B. Das, Tetrahedron Lett., 2009, 50, 897–900 CrossRef CAS.
- P. S. Rao and R. V. Venkataratnam, Tetrahedron Lett., 1991, 32, 5821–5822 CrossRef CAS.
- X.-R. Zhang, W. Chao, Y.-T. Chuai, Y. Ma, R. Hao, D.-C. Zou, Y.-G. Wei and Y. Wang, Org. Lett., 2006, 8, 2563–2566 CrossRef CAS.
- B. Green, R. I. Crane, I. S. Khaidem, R. S. Leighton, S. S. Newaz and T. E. Smyser, J. Org. Chem., 1985, 50, 640–644 CrossRef CAS.
- L. Martins, W. Hoelderich, P. Hammer and D. Cardoso, J. Catal., 2010, 271, 220–227 CrossRef CAS.
- K. M. Parida and D. Rath, J. Mol. Catal. A: Chem., 2009, 310, 93–100 CrossRef CAS.
- K. M. Parida, S. Mallick, P. C. Sahoo and S. K. Rana, Appl. Catal., A, 2010, 381, 226–232 CrossRef CAS.
- G. Li, J. Xiao and W. Zhang, Green Chem., 2011, 13, 1828–1836 RSC.
- B. M. Choudary, M. L. Kantam, P. Sreekanth, T. Bandopadhyay, F. Figueras and A. Tuel, J. Mol. Catal. A: Chem., 1999, 142, 361–365 CrossRef CAS.
- Y. Peng, J. Wang, J. Long and G. Liu, Catal. Commun., 2011, 15, 10–14 CrossRef CAS.
- T. Murase, Y. Nishijima and M. Fujita, J. Am. Chem. Soc., 2012, 134, 162–164 CrossRef CAS.
- S. Saravanamurugan, M. Palanichamy, M. Hartmann and V. Murugesan, Appl. Catal., A, 2006, 298, 8–15 CrossRef CAS.
- M. Srasra, S. Delsarte and E. M. Gaigneaux, Top. Catal., 2009, 52, 1541–1548 CrossRef CAS.
- W. Hu, Z. Guan, X. Deng and Y.-H. He, Biochimie, 2012, 94, 656–661 CrossRef CAS.
- Y.-F. Lai, H. Zheng, S.-J. Chai, P.-F. Zhang and X.-Z. Chen, Green Chem., 2010, 12, 1917–1918 RSC.
- H. Zhao, N. Yu, Y. Ding, R. Tan, C. Liu, D. Yin, H. Qiu and D. Yin, Microporous Mesoporous Mater., 2010, 136, 10–17 CrossRef CAS.
- P. Verdia, F. Santamarta and E. Tojo, Molecules, 2011, 16, 4379–4388 CrossRef CAS.
- D.-Z. Xu, Y. Liu, S. Shi and Y. Wang, Green Chem., 2010, 12, 514–517 RSC.
- Y. Zhang, Y. Zhao and C. Xia, J. Mol. Catal. A: Chem., 2009, 306, 107–112 CrossRef CAS.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and G. Sartori, Green Chem., 2000, 2, 101–103 RSC.
- R. Trotzki, M. M. Hoffmann and B. Ondruschka, Green Chem., 2008, 10, 767–772 RSC.
- J. S. Yadav, D. C. Bhunia, V. K. Singh and P. Srihari, Tetrahedron Lett., 2009, 50, 2470–2473 CrossRef CAS.
- G. Bartoli, M. Bosco, A. Carlone, R. Dalpozzo, P. Galzerano, P. Melchiorre and L. Sambri, Tetrahedron Lett., 2008, 49, 2555–2557 CrossRef CAS.
- Y. Zhang and C. Xia, Appl. Catal., A, 2009, 366, 141–147 CrossRef CAS.
- F. Zhang, Y.-X. Wang, F.-L. Yang, H.-Y. Zhang and Y.-F. Zhao, Synth. Commun., 2011, 41, 347–356 CrossRef CAS.
- D. C. Neckers, D. A. Kooistra and G. W. Green, J. Am. Chem. Soc., 1972, 94, 9284–9285 CrossRef CAS.
- K. P. Borujeni and B. Tamami, Catal. Commun., 2007, 8, 1191–1196 CrossRef CAS.
- B. Tamami and K. P. Borujeny, Tetrahedron Lett., 2004, 45, 715–718 CrossRef CAS.
- A. P. Deshmukh, K. J. Padiya and M. M. Salunkhe, J. Chem. Res. (S), 1999, 568–569 RSC.
- A. Rahman, G. Lemay, A. Adnot and S. Kaliaguine, J. Catal., 1988, 112, 453–463 CrossRef CAS.
- A. Marrone, A. Renzetti, P. De Maria, S. Gerard, J. Sapi, A. Fontana and N. Re, Chem.–Eur. J., 2009, 15, 11537–11550 CrossRef CAS.
- J. Barluenga, L. Riesgo, R. Vicente, L. A. Lopez and M. Tomas, J. Am. Chem. Soc., 2007, 129, 7772 CrossRef CAS.
- W.-B. Yi, Y.-Q. Yin and C. Cai, Org. Prep. Proced. Int., 2007, 39, 71–75 CrossRef CAS.
- R. Qiu, Y. Qiu, S. Yin, X. Song, Z. Meng, X. Xu, X. Zhang, S. Luo, C.-T. Au and W.-Y. Wong, Green Chem., 2010, 12, 1767–1771 RSC.
- E. Angeletti, C. Canepa, G. Martinetti and P. Venturello, J. Chem. Soc., Perkin Trans. 1, 1989, 105–107 RSC.
- Y. Zhang and C. Xia, Appl. Catal., A, 2009, 366, 141–147 CrossRef CAS.
- W.-B. Yi and C. Cai, Catal. Commun., 2008, 9, 1291–1296 CrossRef CAS.
- J. S. Yadav, D. C. Bhunia, V. K. Singh and P. Srihari, Tetrahedron Lett., 2009, 50, 2470–2473 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra, 13C NMR spectrum, GC-MS profile, HRMS profile. See DOI: 10.1039/c2ra21571c |
| This journal is © The Royal Society of Chemistry 2012 |