Tandem 1,5-migration/Michael reactions to prepare adducts of pyrazolone derivatives: protecting group-directed rearrangement†
Zhijin
Lu
a,
Chen
Xie
a,
Jianlin
Han
*a and
Yi
Pan
*ab
aSchool of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, China. E-mail: hanjl@nju.edu.cn; Fax: +86-25-83592846; Tel: +86-25-83593153
bState of Key Laboratory of Coordination, Nanjing University, Nanjing, 210093, China. E-mail: yipan@nju.edu.cn
First published on 15th August 2012
Abstract
A tandem 1,5-migration/Michael reaction of pyrazoline has been described, which results in different multiply-substituted pyrazolone derivatives with excellent yields controlled by protecting group.
In recent years, migration reactions have received much attention.1 Particularly, migration reactions in tandem processes have led to the development of diverse and versatile synthetic methods.2 Migration occurs in some organic reactions, which may be of value or a disadvantage for the strategies desired in synthesis.3 So, control of the stereochemistry is of high interest and is still a challenging problem in migration processes, especially for the stereoselective construction of molecules with remote centers.4 Furthermore, this moiety usually exists in many heterocyclic natural products and medicines.5 Generally, the migration can be observed under different reaction conditions.6 On the other hand, this isomerization occurs in the reaction of compounds with dramatically different core structures.7 However, the control of the remote migration reaction with a similar core structure under the same reaction conditions remains unexplored.
Pyrazolone derivatives, representing an important class of five-membered-ring lactams, have exhibited a variety of applications as pharmaceutical agents,8 synthetic scaffolds in combinatorial and medicinal chemistry.9 Especially, pyrazolin-5-ones derivatives have been well known as analgesic and antipyretic agents.10 In this respect, the development of a facile and excellent strategy for the synthesis of new pyrazolin-5-ones derivatives seems to be attractive and highly interesting. However, the reactions of pyrazolin-5-ones still have not been well documented.11 In particular, there was only one example about the pyrazolin-5-ones used as precursors in a tandem process.12 Herein, we reported a facile tandem 1,5-migration/Michael reaction of 3-substituted pyrazolines with nitroalkenes. The resultant different pyrazoline derivatives were obtained through different processes controlled by the protecting group on the oxygen atom of pyrazolines under the same reaction conditions. The current reaction also provides an easy access to multiply-substituted pyrazolin-5-one derivatives from readily available starting materials.
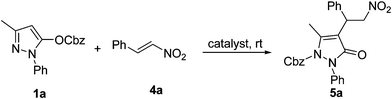
Initially, we used pyrazoline 1a and nitrostyrene 4a as model substrates with DMAP as catalyst. We assumed that the reaction proceeds through the designed route, as shown in pathway a in Scheme 1. However, no desired adducts were obtained from the reaction after 12 h. Surprisingly, the reaction was found to proceed through pathway b, and resulted in compound 5a with 95% yield. This suggested that the reagent 1a undergoes the arrangement in the presence of Lewis base, and the benzyloxycarbonyl protecting group migrated from an oxygen atom to a nitrogen atom. A similar rearrangement was also found in the previous report with Lewis acid as catalyst.13
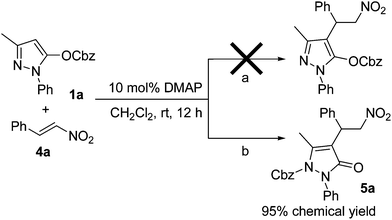 | ||
| Scheme 1 Reaction of pyrazoline 1a with nitrostyrene 4a. | ||
To improve the reaction efficiency, several other catalysts were used for the reaction (Table 1). No reaction was detected at all when no catalyst was used in the reaction (entry 2). Although other base catalysts also could promote the reaction, the resulting yields were dramatically lower (entries 3–8). Then, the optimization with DMAP as catalyst was focused on the screening of solvent, and CH2Cl2 was found to be the best choice. Notably, when acetonitrile or acetone was employed in this reaction, the reaction time was much longer with a dramatic decrease in yields (entries 12–13). The further optimization found that the catalyst loading amount could be lowered to 1 mol% without any appreciable drop in reactivity and yield after 12 h (entry 15). However, when the catalyst loading increased to 50%, the corresponding adduct 5a was obtained with only 28% yield (entry 16).
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Amount (mol%) | Solvent | Time (h) | Yield (%)c |
| a Reaction conditions: 1a (0.5 mmol), 4a (0.75 mmol), solvent (2 mL) at room temperature. b Tetramethylguanidine. c Isolated yields. | |||||
| 1 | DMAP | 10 | CH2Cl2 | 12 | 95 |
| 2 | No | — | CH2Cl2 | 24 | NR |
| 3 | DIPEA | 10 | CH2Cl2 | 24 | 28 |
| 4 | DABCO | 10 | CH2Cl2 | 72 | 34 |
| 5 | DBU | 10 | CH2Cl2 | 72 | 45 |
| 6 | TMGb | 10 | CH2Cl2 | 72 | 32 |
| 7 | Et3N | 10 | CH2Cl2 | 24 | 21 |
| 8 | Pyridine | 10 | CH2Cl2 | 24 | 17 |
| 9 | DMAP | 10 | CHCl3 | 12 | 78 |
| 10 | DMAP | 10 | DME | 12 | 92 |
| 11 | DMAP | 10 | Toluene | 12 | 80 |
| 12 | DMAP | 10 | Acetone | 72 | 48 |
| 13 | DMAP | 10 | CH3CN | 72 | 45 |
| 14 | DMAP | 5 | CH2Cl2 | 12 | 95 |
| 15 | DMAP | 1 | CH2Cl2 | 12 | 92 |
| 16 | DMAP | 50 | CH2Cl2 | 12 | 28 |
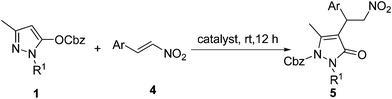
After the optimized condition was established, we began to examine the substrate scope of the tandem reaction with pyrazolines 1 and nitroolefins 4 (Table 2). Generally, the reaction could proceed smoothly to generate multiply-substituted pyrazolin-5-one derivatives. For all the probed nitroolefins, those with one halogen on the aromatic ring could participate better in the reaction (entries 3–5), resulting in the desired products with higher yields (92%–95%). Contrasting with electronic features, steric features have a greater effect on the chemical yields. The reactions gave sharply lower yields when the phenyl ring was changed to a 1-naphthyl ring (81% yield, entry 8). More significantly, the reaction of the substrates with a heteroaromatic ring also proceeded well, affording the desired product with good yields (entries 9–10). We further checked the effects of substituents on the nitrogen atom of the pyrazolines on this reaction. To our delight, the reaction also proceeded well to give the desired products in excellent yields (entries 11–13). Results in Table 2 disclosed that the variation of R1 substituent on pyrazolines and aromatic ring of nitrostyrenes was not the key factor for the migration.
|
|
||||
|---|---|---|---|---|
| Entry | Ar | R1 | Product | Yield (%)b |
| a Reaction conditions: 1 (0.5 mmol), 4 (0.75 mmol), solvent (2 mL) at room temperature. b Isolated yields. | ||||
| 1 | C6H5 | C6H5 | 5a | 92 |
| 2 | 4-MeC6H4 | C6H5 | 5b | 92 |
| 3 | 2-FC6H4 | C6H5 | 5c | 92 |
| 4 | 4-ClC6H4 | C6H5 | 5d | 95 |
| 5 | 4-BrC6H4 | C6H5 | 5e | 95 |
| 6 | 4-OMeC6H4 | C6H5 | 5f | 92 |
| 7 | 3-OMeC6H4 | C6H5 | 5g | 95 |
| 8 | 1-Naphthyl | C6H5 | 5h | 81 |
| 9 | 2-Furyl | C6H5 | 5i | 83 |
| 10 | 2-Thienyl | C6H5 | 5j | 76 |
| 11 | C6H5 | 4-ClC6H4 | 5k | 92 |
| 12 | 2-FC6H4 | 4-ClC6H4 | 5l | 93 |
| 13 | 2-ClC6H4 | 4-ClC6H4 | 5m | 94 |
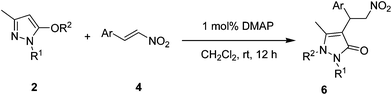
To investigate the cause for the 1,5-migration, reactions of pyrazolines with other usual alkoxycarbonyl protecting groups were performed under the optimized conditions (Table 3). After careful characterizations, we found that the alkoxycarbonyl protecting group also migrated from the oxygen atom to the nitrogen atom for all cases. We were also glad to see that the reactions could proceed smoothly, and resulted in excellent yields for all the nitrostyrene derivatives within 12 h. In addition, heterocyclic substrates also reacted well with pyrazolines to give the desired products with slightly lower yields (entries 8 and 9). The absolute stereochemistry of these products was confirmed by single-crystal X-ray analysis of 6j (Fig. 1).
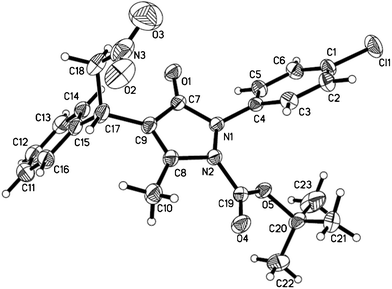 | ||
| Fig. 1 ORTEP diagram showing of compound 6j. | ||
|
|
|||||
|---|---|---|---|---|---|
| Entry | Ar | R1 | R2 | Product | Yield (%)b |
| a Reaction conditions: pyrazolines 2 (0.5 mmol), nitroolefin 4 (0.75 mmol), CH2Cl2 (2 mL), 1 mol% DMAP. b Isolated yields. | |||||
| 1 | C6H5 | C6H5 | Boc | 6a | 90 |
| 2 | 4-FC6H4 | C6H5 | Boc | 6b | 92 |
| 3 | 4-ClC6H4 | C6H5 | Boc | 6c | 94 |
| 4 | 4-BrC6H4 | C6H5 | Boc | 6d | 98 |
| 5 | 4-OMeC6H4 | C6H5 | Boc | 6e | 98 |
| 6 | 2-ClC6H4 | C6H5 | Boc | 6f | 96 |
| 7 | 1-Naphthyl | C6H5 | Boc | 6g | 82 |
| 8 | 2-Furyl | C6H5 | Boc | 6h | 65 |
| 9 | 2-Thienyl | C6H5 | Boc | 6i | 70 |
| 10 | C6H5 | 4-ClC6H4 | Boc | 6j | 94 |
| 11 | 4-ClC6H4 | 4-ClC6H4 | Boc | 6k | 95 |
| 12 | 4-BrC6H4 | 4-ClC6H4 | Boc | 6l | 92 |
| 13 | C6H5 | C6H5 | EtOCO | 6m | 98 |
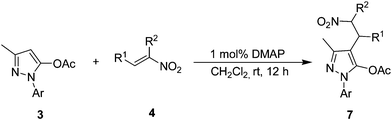
Then we continued to scan the reaction parameters for the key factor for the migration. We used the pyrazol-5-yl acetates 3 as substrates to perform the reaction under the optimized condition (entry 1, Table 4). Surprisingly, when the nitrogen protecting group was changed into acetyl, the starting materials were totally converted into the regular Michael product and no migration product was detected. Notably, the similar products were found for all probed nitroolefins with high yields. The chemical structures of these no migration products have been confirmed by single-crystal X-ray analysis of 7h (Fig. 2). As the results show in Table 4, the variation of the protecting group of pyrazolines directly led to different products, and the migration did not occur during the process.
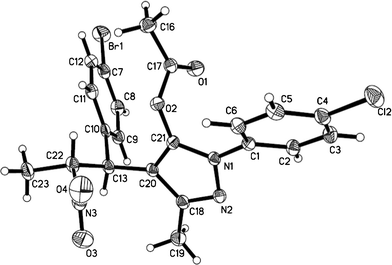 | ||
| Fig. 2 ORTEP diagram showing of compound 7h. | ||
|
|
|||||
|---|---|---|---|---|---|
| Entry | Ar | R1 | R2 | Product | Yield (%)b |
| a Reaction conditions: pyrazol-5-yl-acetate 3 (0.5 mmol), nitroolefin 4 (0.75 mmol), CH2Cl2 (2 mL), 1 mol% DMAP. b Isolated yields. c DBU was used as catalyst. | |||||
| 1 | C6H5 | C6H5 | H | 7a | 95 |
| 2 | C6H5 | 2-ClC6H4 | H | 7b | 92 |
| 3 | C6H5 | 4-ClC6H4 | H | 7c | 94 |
| 4 | C6H5 | 4-MeC6H4 | H | 7d | 73 |
| 5 | C6H5 | 2-FC6H4 | H | 7e | 82 |
| 6 | C6H5 | 3-FC6H4 | H | 7f | 88 |
| 7 | C6H5 | 4-FC6H4 | H | 7g | 83 |
| 8 | 4-ClC6H4 | 4-BrC6H4 | Me | 7h | 52c |
On the basis of the above results, a tandem migration/Michael mechanism has been proposed for the current process as shown in Scheme 2. The initial step is the DMAP-catalyzed generation of enolate A. Then there is the Michael addition of intermediate A to nitrostyrene, giving the intermediate B, which also exists as tautomeric form C. The intermediate C undergoes an O-carboxylation process to form the no migration adducts 7, when the R group is a methyl. However, the reaction will proceed through N-carboxylation to give the migration adducts 5 or 6, when the R group is an alkoxy group. A similar process could be found in the previous report from Fu's group.14
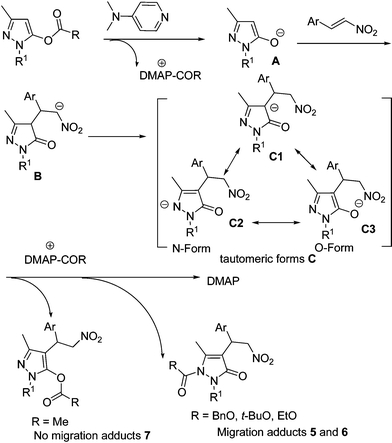 | ||
| Scheme 2 The proposed mechanism. | ||
Conclusions
In summary, we developed a facile and highly efficient tandem 1,5-migration/Michael reaction of pyrazolines to nitroolefins, resulting in different multiply 4-substituted-5-pyrazolone derivatives. The protecting group in pyrazolines controlled the migration, to give the different adducts with excellent yields. The present reaction provides a straightforward solution to the challenging problem of generating pyrazolone derivatives. Studies exploring the asymmetric manner for this reaction with organocatalysts are actively underway in our laboratory.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21102071) and the Fundamental Research Funds for the Central Universities (No. 1107020522 and No. 1082020502). The Jiangsu 333 program (for Pan) and Changzhou Jin-Feng-Huang program (for Han) are also acknowledged.References
- (a) For papers on migration reaction, see: N. Dieltiens, C. V Stevens, K. G. R. Masschelein and T. Rammeloo, Tetrahedron, 2005, 61, 6749–6756 CrossRef CAS; (b) K. W. Kells, A. Ncube and J. M. Chong, Tetrahedron, 2004, 60, 2247–2257 CrossRef CAS; (c) F. Xu, W. F. Shi and J. B. Wang, J. Org. Chem., 2005, 70, 4191–4194 CrossRef CAS; (d) W. F. Shi, F. P. Xiao and J. B. Wang, J. Org. Chem., 2005, 70, 4318–4322 CrossRef CAS; (e) S. Ishida, T. Iwamoto and M. Kira, Organometallics, 2009, 28, 919–921 CrossRef CAS; (f) D. Cantillo, M. Ávalos, R. Babiano, P. Cintas, J. L. Jiménez, M. E. Light and J. C. Palacios, J. Org. Chem., 2010, 75, 4300–4303 CrossRef CAS; (g) L. Mouls, G. Subra, C. Enjalbal, J. Marinez and J. L. Aubagnac, Tetrahedron Lett., 2004, 45, 1173–1178 CrossRef CAS; (h) S. G érard and J. Marchand-Brynaert, Tetrahedron Lett., 2003, 44, 6339–6342 CrossRef; (i) K. C. Majumdar, S. Hazra and B. Roy, Tetrahedron Lett., 2011, 52, 6697–6701 CrossRef CAS; (j) Q. H. Huang, M. A. Campo, T. Yao, Q. P. Tian and R. C. Larock, J. Org. Chem., 2004, 69, 8251–8257 CrossRef CAS.
- (a) W. D. Rao, D. Susanti and P. W. H. Chan, J. Am. Chem. Soc., 2011, 133, 15248–15251 CrossRef CAS; (b) H. J. Zheng, J. Y. Zheng, B. X. Yu, Q. Chen, X. L. Wang, Y. P. He, Z. Yang and X. G. She, J. Am. Chem. Soc., 2010, 132, 1788–1789 CrossRef CAS; (c) K. Tanaka, E. Okazaki and Y. Shibata, J. Am. Chem. Soc., 2009, 131, 10822–10823 CrossRef CAS; (d) D. Leboeuf, A. Simonneau, C. Aubert, M. Malacria, V. Gandon and L. Fensterbank, Angew. Chem., Int. Ed., 2011, 50, 6868–6871 CrossRef CAS; (e) T. Matsuda, Y. Suda and A. Takahashi, Chem. Commun., 2012, 48, 2988–2990 RSC; (f) S. Y. Huang, X. X. Li, C. L. Lin, L. A. Guzei and W. P. Tang, Chem. Commun., 2012, 48, 2204–2206 RSC; (g) M. D. Swift and A. Sutherland, Org. Lett., 2007, 9, 5239–5242 CrossRef CAS; (h) A. M. Fournier and J. Clayden, Org. Lett., 2012, 14, 142–145 CrossRef CAS.
- (a) R. G. Soengas, J. C. Estévez and R. J. Estévez, Tetrahedron, 2003, 59, 6285–6289 CrossRef CAS; (b) D. J. Tetlow, U. Hennecke, J. Raftery, M. J. Waring, D. S. Clarke and J. Clayden, Org. Lett., 2010, 12, 5442–5445 CrossRef CAS; (c) K. G. Ji, X. Z. Shu, J. Chen, S. C. Zhao, Z. J. Zheng, L. Lu, X. Y. Liu and Y. M. Liang, Org. Lett., 2008, 10, 3919–3922 CrossRef CAS.
- (a) A. Menzek and A. Altundas, Tetrahedron, 2006, 62, 12318–12325 CrossRef CAS; (b) M. Alajarin, M. M. Ortin, P. Sanchez-Andrada and A. Vidal, J. Org. Chem., 2006, 71, 8126–8139 CrossRef CAS; (c) R. Koch, M. W. Wong and C. Wentrup, J. Org. Chem., 1996, 61, 6809–6813 CrossRef CAS.
- (a) R. H. Wiley and P. Wiley, Pyrazolones, Pyrazolidones and Derivatives, ed. A. Weissbergerinterscience, New York, 1964 Search PubMed; (b) K. Bruce, Agents and Actions supplements. 100 years of Pyrazolone Drugs, An Update, ed. K. Bruce, Birkhäuser Verlag: Basel, Switzerland, 1986 Search PubMed; (c) K. C. Majumdar and S. K. Chattopadhyay, Heterocycles in Natural Product Synthesis, Wiley, New York, 2011 Search PubMed; (d) L. I. Blenkii, V. N. Gramenitskaya and Y. B. Evdokimenkova, Advances in Heterocyclic Chemistry, 2011 Search PubMed; (e) M. Brasholz, H. U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45–56 CrossRef CAS; (f) G. Negri, C. Kascheres and A. J. Kascheres, J. Heterocycl. Chem., 2004, 41, 461–491 CrossRef CAS.
- (a) V. A. Barkhash, Top. Curr. Chem., 1984, 116/117, 1–265 CrossRef; (b) S. H. Pine, J. B. Hendrickson, D. J. Cram and G. S. Hammond, Organic Chemistry, 4th edn; McGraw-Hill International Book Company, Tokyo, 1982 Search PubMed; (c) F. Pünner, A. Schmidt and G. Hilt, Angew. Chem., Int. Ed., 2012, 51, 1270–1273 CrossRef; (d) R. Uma, C. Crévisy and R. Grée, Chem. Rev., 2003, 103, 27–52 CrossRef CAS.
- (a) Y. B. Nie, L. Wang and M. W. Ding, J. Org. Chem., 2012, 77, 696–700 CrossRef CAS; (b) M. Mondon, N. Fontelle, J. Désiré, F. Lecornué, J. Guillard, J. Marrot and Y. Blériot, Org. Lett., 2012, 14, 870–873 CrossRef CAS; (c) N. Okamoto, K. Takeda and R. Yanada, J. Org. Chem., 2010, 75, 7615–7625 CrossRef CAS.
- (a) N. Yokoyama, B. Ritter and A. D. Neubert, J. Med. Chem., 1982, 25, 337–339 CrossRef CAS; (b) R. I. Fryer, P. Zhang, R. Rios, Z. Q. Gu, A. S. Basile and P. Skolnick, J. Med. Chem., 1993, 36, 1669–1673 CrossRef CAS; (c) L. Savini, P. Massarelli, C. Nencini, C. Pellerano, G. Biggio, A. Maciocco, G. Tuligi, A. Carrieri, N. Cinone and A. Carotti, Bioorg. Med. Chem., 1998, 6, 389–399 CrossRef CAS; (d) M. G. Ferlin, G. Chiarelotto, S. D. Acqua, E. Maciocco, M. P. Mascia, M. G. Pisu and G. Biggio, Bioorg. Med. Chem., 2005, 13, 3531–3541 CrossRef CAS; (e) A. Kimata, H. Nakagawa, R. Ohyama, T. Fukuuchi, S. Ohta, T. Suzuki and N. Miyata, J. Med. Chem., 2007, 50, 5053–5056 CrossRef CAS.
- (a) O. A. Attanasi, P. Filippone, B. Guidi, T. Hippe, F. Mantellini and L. F. Tietze, Tetrahedron Lett., 1999, 40, 9277–9280 CrossRef CAS; (b) F. Lehmann, M. Holm and S. Laufer, J. Comb. Chem., 2008, 10, 364–367 CrossRef CAS; (c) F. Caruso, M. Rossi, J. Tanski, R. Sartori, R. Sariego, S. Moya, S. Diez, E. Navarrete, A. Cingolani, F. Marchetti and C. Pettinari, J. Med. Chem., 2000, 43, 3665–3670 CrossRef CAS; (d) F. Caruso, C. Pettinari, F. Marchetti, P. Natanti, C. Phillips, J. Tanski and M. Rossi, Inorg. Chem., 2007, 46, 7553–7560 CrossRef CAS.
- (a) T. Watanabe, S. Yuki, M. Egawa and H. J. Nishi, Pharmacol. Exp. Ther., 1994, 268, 1597–1604 CAS; (b) H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and K. I. J. Saito, Pharmacol. Exp. Ther., 1997, 281, 921–927 CAS; (c) A. Graul and J. Castaner, Drugs Future, 1996, 21, 1014–1016 CAS; (d) A. S. Galabov, A. V.Terebenina, K. Dimitrova, O. Todorova, A. Karparov and G. Borisov, Dokl. Bolg. Akad. Nauk., 1990, 43, 61 CAS.
- (a) Y. H. Liao, W. B. Chen, Z. J. Wu, X. L. Du, L. F. Cun, X. M. Zhang and W. C. Yuan, Adv. Synth. Catal., 2010, 352, 827–832 CrossRef CAS; (b) Z. Wang, Z. G. Yang, D. H. Chen, X. H. Liu, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2011, 50, 4928–4932 CrossRef CAS; (c) Z. G. Yang, Z. Wang, S. Bai, X. H. Liu, L. L. Lin and X. M. Feng, Org. Lett., 2011, 13, 596–599 CrossRef CAS.
- A. N. R. Alba, A. Zea, G. Valero, T. Calbet, M. Font-Bardía, A. Mazzanti, A. Moyano and R. Rios, Eur. J. Org. Chem., 2011, 1318–1325 CrossRef CAS.
- H. Maruoka, K. Yamagata, F. Okabe and Y. Tomioka, J. Heterocycl. Chem., 2006, 43, 859–865 CrossRef CAS.
- J. C. Ruble and G. C. Fu, J. Am. Chem. Soc., 1998, 120, 11532–11533 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental procedures, full spectroscopic data for new compounds and single-crystal X-ray diffraction analysis of 2a, 6j and 7h. CCDC reference numbers 861353, 874014 and 874398. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra21549g |
| This journal is © The Royal Society of Chemistry 2012 |