Protic ionic liquids: an alternative proton-conducting electrolyte for high temperature proton exchange membrane fuel cells†
Changchun
Ke
ab,
Jin
Li
ab,
Xiaojin
Li
*a,
Zhigang
Shao
*a and
Baolian
Yi
a
aDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Zhongshan Road 457, Dalian, 116023, China. E-mail: xjli@dicp.ac.cn; zhgshao@dicp.ac.cn
bGraduate University of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 13th August 2012
Abstract
A novel anhydrous proton-conductive membrane, composed of an ionic liquid tertiary amine phosphate as the electrolyte and polypropylene-nonwoven as the matrix, was first proposed and applied in high temperature proton exchange membrane fuel cells. The single cell fabricated with the PP-NW/[N111]·H2PO4 composite membrane gives a stable current density of 600 mA cm−2 under 140 °C and non-humidification conditions, making the proton exchange membranes based on ionic liquids much more prospective for high temperature proton exchange membrane fuel cells.
Operation of proton exchange membrane fuel cells (PEMFCs) at high temperature (>100 °C) has many advantages, such as higher CO durability, simple water management and humidifier exemption and so on. Therefore, the R&D of proton exchange membranes (PEMs) for working above 100 °C is one of the most important issues in the PEMFC research field. A lot of efforts have been made to enable commercialized Nafion® (DuPont, USA) to work under the conditions of non-humidification and high temperature. However, the modified Nafion composite membranes, such as Nafion/SiO2, Nafion/TiO2, Nafion/ZrP developed in recent years, cannot match the requirements of high temperature proton exchange membrane fuel cells (HT-PEMFCs) well, and no breakthrough has yet been achieved.1 At present, PEMFCs using modified Nafion as the electrolyte still require external humidification, and the working temperature is limited to less than 140 °C, normally under 120 °C. This working temperature cannot highlight the advantages of HT-PEMFC, for example, higher CO durability, etc.2 Therefore, to further elevate the working temperature of PEMFCs to above 140 °C is the aim of the development of proton conducting membranes. Acid–base complexation represents an effective approach to the above requirements.3 A breakthrough was achieved when polybenzimidazole (PBI) was first proposed for preparing acid-doped membranes.4–6 Besides, solid acids (e.g., CsH2PO4), are known to undergo a structural change which leads to a highly conductive superprotonic phase at temperatures above 140 °C.7
Room temperature ionic liquids (RTILs), are relatively new to chemistry and the chemical sciences, and are very promising for developing novel anhydrous proton-conductive membranes for HT-PEMFCs.8 In the past decade, a series of literature studies focusing on RTILs as the electrolyte system have been reported.9–12 Most of them have not provided the experimental data of the single cell performance based on ionic liquid PEMs.13–16 A number of the papers concerning the cell performance give the highest stable current density at no more than 10 mA cm−2.17–19 Recently, researchers from Professor Masayoshi Watanabe's group, have used a novel electrolyte system derived from [dema]·[TfO] and sulfonated polyimide (SPI).20 The results show that the [dema]·[TfO]/SPI composite membrane has a good cell performance without humidification at 30 °C. However, when the temperature of 140 °C arises, the performance decreases a lot due to the volatilization of the residual water in the ionic liquid. Ref. 20 reported the highest stable current density, of about 100 mA cm−2, at the output potential of 0.1 V, which is the best cell performance of PEMs based on ionic liquids till now.21
In this paper, we report a novel anhydrous proton-conductive membrane composed of an ionic liquid tertiary amine phosphate (marked as [N111]·H2PO4, structural formula shown in Fig. 1) as the electrolyte and polypropylene-nonwoven (referred to as “PP-NW” hereinafter) as the matrix. This electrolyte system has a high loading of [N111]·H2PO4 in PP-NW, as high as 1800 wt%. The composite membrane exhibits a much higher proton conductivity with a thickness of 120 μm. The single cell fabricated with the PP-NW/[N111]·H2PO4 composite membrane was further investigated. For this purpose, the H2/O2 fuel cell prepared using the PP-NW/[N111]·H2PO4 composite membrane was operated at 130 °C and 140 °C without humidification. The polarization curves of the cell fabricated with the PP-NW/[N111]·H2PO4 membrane are shown in Fig. 1. A current density higher than 600 mA cm−2 is achieved at 140 °C, which is much higher than the reported ionic liquid systems.22,23 Besides, the PP-NW/[N111]·H2PO4 membrane shows good stability according to the open circuit voltage (OCV) decay acceleration test, as shown in Fig. 2.
![Polarization curves of single PEM fuel cell equipped with [N111]·H2PO4/PP-NW operated at various conditions.](/image/article/2012/RA/c2ra21378h/c2ra21378h-f1.gif) | ||
| Fig. 1 Polarization curves of single PEM fuel cell equipped with [N111]·H2PO4/PP-NW operated at various conditions. | ||
![OCV–time curve of the OCV decay acceleration test of PP-NW/[N111]·H2PO4.](/image/article/2012/RA/c2ra21378h/c2ra21378h-f2.gif) | ||
| Fig. 2 OCV–time curve of the OCV decay acceleration test of PP-NW/[N111]·H2PO4. | ||
In our laboratory, we also made attempts to develop other electrolyte systems based on ionic liquids, including the most intensively investigated pyridine and imidazole based ionic liquids with high proton conductivity. However, cells fabricated with those electrolyte systems, e.g., [BMIM]·H2PO4, [EMIM]·HSO4, can only generate a highest current density of no more than 10 mA cm−2 (as shown in Fig. 3), the same as the results of many literature studies.20,24–26 In order to explain this phenomenon, CV curves of blank Pt/C and Pt/C electrodes dyed with ionic liquids were investigated. The results are shown in Fig. 4. It is clearly seen that the pyridine and imidazole based ionic liquids strongly poisons the Pt/C catalyst. Whereas, the [N111]·H2PO4 ionic liquid shows much less poisoning of the Pt/C catalyst, in comparison with pyridine and imidazole based ionic liquids. It is concluded that the compatibility between ionic liquids and the catalysts of PEMFCs has a great effect on the single cell performance.
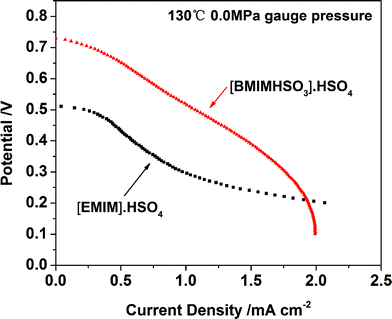 | ||
| Fig. 3 Typical polarization curves of the PEMFC single cell equipped with membranes based on imidazole ionic liquids and Nafion hybrid membranes. | ||
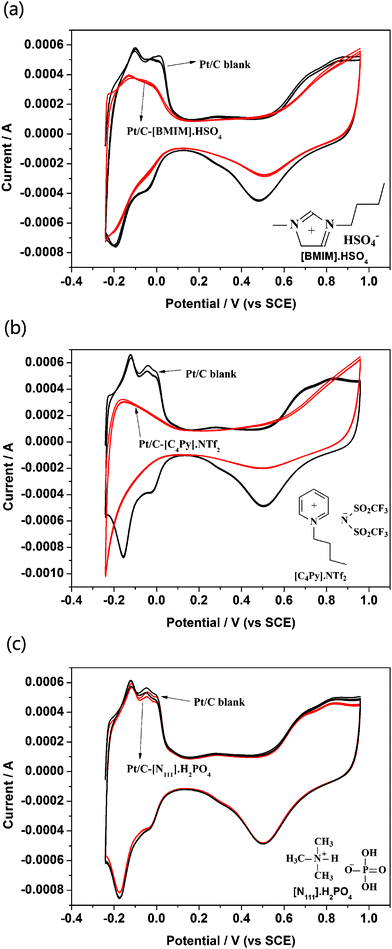 | ||
| Fig. 4 CV curves of Pt/C electrodes dyed with several types of ionic liquids investigated in our laboratory, in comparison with blank Pt/C. | ||
The other important factor responsible for the cell performance is the loading content of the ionic liquid to the neat matrix. As the matrix, PP-NW has a high loading content of ionic liquid, as mentioned above. The ionic conductivity of the PP-NW/[N111]·H2PO4 membrane was further investigated at different temperatures. Fig. 5 gives the Arrhenius plot of the ionic conductivities of [N111]·H2PO4 ionic liquid and PP-NW/[N111]·H2PO4 composite membranes. Although the conductivity of [N111]·H2PO4 is not very highlighted in comparison with the pyridine and imidazole based ionic liquids, the conductivity of PP-NW/[N111]·H2PO4 membrane is higher than 0.016 S cm−1 at 160 °C, as high as 50–60% of that of the [N111]·H2PO4 ionic liquid. In comparison, ref. 20 reported that the highest loading of the ionic liquid [dema]·[TfO] is 80 wt% to SPI, and that the ionic conductivity of the [dema]·[TfO]/SPI composite membrane is in magnitude of 10−3 S cm−1 with 67 wt% [dema]·[TfO], which is much lower than that of pure [dema]·[TfO] (∼10−1 S cm−1).
![Ionic conductivities of [N111]·H2PO4 and the [N111]·H2PO4/PP-NW hybrid membrane.](/image/article/2012/RA/c2ra21378h/c2ra21378h-f5.gif) | ||
| Fig. 5 Ionic conductivities of [N111]·H2PO4 and the [N111]·H2PO4/PP-NW hybrid membrane. | ||
The high ionic conductivity of the PP-NW/[N111]·H2PO4 composite membrane can be well explained by the effective media theory and the high void fraction of PP-NW. From the FE-SEM image of neat PP-NW shown in Fig. 6(a), the high void structure of the nonwoven matrix can be seen. From the FE-SEM image of the PP-NW/[N111]·H2PO4 composite membrane, it can be seen that the void pore in the PP-NW is filled with ionic liquid. According to the effective media theory proposed by Bruggeman in the 1930s,27,28 the conductivity of the composite electrolyte, σh, can be calculated by eqn (1).29
| σh = σ1(f − (1 − f)/A)t | (1) |
![FE-SEM images of the neat PP-NW (a) and PP-NW/[N111]·H2PO4 hybrid membrane (b).](/image/article/2012/RA/c2ra21378h/c2ra21378h-f6.gif) | ||
| Fig. 6 FE-SEM images of the neat PP-NW (a) and PP-NW/[N111]·H2PO4 hybrid membrane (b). | ||
In eqn (1), σh stands for the overall conductivity of the composite membrane, σ1 stands for conductivity of the effective component. A and t are constants dependent on the chemical composition and structure of the effective component and the matrix, and f is the volume fraction of the matrix. It is clear that if the fraction f is enhanced, the overall conductivity of the composite membrane will increase according to the index function. So the fraction of the potential volume in the matrix is very important, as it determines the overall conductivity of the composite membrane.
Conclusions
In conclusion, a new anhydrous proton conductive membrane derived from PP-NW and [N111]·H2PO4 was first proposed. This electrolyte system can work well under high temperatures and non-humidification conditions, and so far gives the best cell performances based on protic ionic liquids, providing a successful case for ionic liquid based membranes for HT-PEMFCs. This electrolyte system makes the proton exchange membranes based on ionic liquids much more prospective. In the very near future, further work will be done on the transport processes of ions in this proton-conducting membrane to increase cell performance and OCV.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21076210 and 20936008). We also thank Dr Lixing Hao for the provision of the polypropylene-nonwoven.References
- C. H. Park, C. H. Lee, M. D. Guiver and Y. M. Lee, Prog. Polym. Sci., 2011, 36, 1443–1498 CrossRef CAS.
- K. T. Adjemian, S. J. Lee, S. Srinivasan, J. Benziger and A. B. Bocarsly, J. Electrochem. Soc., 2002, 149, A256–A261 CrossRef CAS.
- Q. F. Li, J. O. Jensen, R. F. Savinell and N. J. Bjerrum, Prog. Polym. Sci., 2009, 34, 449–477 CrossRef CAS.
- J. S. Wainright, J. T. Wang, D. Weng, R. F. Savinell and M. Litt, J. Electrochem. Soc., 1995, 142, L121–L123 CrossRef CAS.
- H. Steininger, M. Schuster, K. D. Kreuer, A. Kaltbeitzel, B. Bingol, W. H. Meyer, S. Schauff, G. Brunklaus, J. Maier and H. W. Spiess, Phys. Chem. Chem. Phys., 2007, 9, 1764–1773 RSC.
- C. H. Park, C. H. Lee, M. D. Guiver and Y. M. Lee, Prog. Polym. Sci., 2011, 36, 1443–1498 CrossRef CAS.
- S. M. Haile, P. M. Calkins and D. Boysen, Solid State Ionics, 1997, 97, 145–151 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS.
- M. Doyle, S. K. Choi and G. Proulx, J. Electrochem. Soc., 2000, 147, 34–37 CrossRef CAS.
- L. A. Neves, J. Benavente, I. M. Coelhoso and J. G. Crespo, J. Membr. Sci., 2010, 347, 42–52 CrossRef CAS.
- S. S. Sekhon, J. S. Park, J. S. Baek, S. D. Yim, T. H. Yang and C. S. Kim, Chem. Mater., 2010, 22, 803–812 CrossRef CAS.
- M. K. Mistry, S. Subianto, N. R. Choudhury and N. K. Dutta, Langmuir, 2009, 25, 9240–9251 CrossRef CAS.
- S. S. Sekhon, J. S. Park, E. Cho, Y. G. Yoon, C. S. Kim and W. Y. Lee, Macromolecules, 2009, 42, 2054–2062 CrossRef CAS.
- A. Fernicola, M. A. Navarra and S. Panero, J. Appl. Electrochem., 2008, 38, 993–996 CrossRef CAS.
- Z. Y. Li, Q. Zhang, H. T. Liu, P. He, X. D. Xu and J. H. Li, J. Power Sources, 2006, 158, 103–109 CrossRef CAS.
- A. Fernicola, S. Panero and B. Scrosati, J. Power Sources, 2008, 178, 591–595 CrossRef CAS.
- G. Lakshminarayana, M. Nogami and I. V. Kityk, J. Electrochem. Soc., 2010, 157, B892–B899 CrossRef CAS.
- H. Nakamoto and M. Watanabe, Chem. Commun., 2007, 2539–2541 RSC.
- G. Lakshminarayana and M. Nogami, Electrochim. Acta, 2010, 55, 1160–1168 CrossRef CAS.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764–9773 CrossRef CAS.
- R. F. de Souza, J. C. Padilha, R. S. Goncalves and J. Dupont, Electrochem. Commun., 2003, 5, 728–731 CrossRef CAS.
- S. S. Sekhon, P. Krishnan, B. Singh, K. Yamada and C. S. Kim, Electrochim. Acta, 2006, 52, 1639–1644 CrossRef CAS.
- B. S. Lalia, K. Yamada, M. S. Hundal, J. S. Park, G. G. Park, W. Y. Lee, C. S. Kim and S. S. Sekhon, Appl. Phys. A: Mater. Sci. Process., 2009, 96, 661–670 CrossRef CAS.
- J. S. Lee, T. Nohira and R. Hagiwara, J. Power Sources, 2007, 171, 535–539 CrossRef CAS.
- A. Noda, A. B. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- H. Nakamoto, A. Noda, K. Hayamizu, S. Hayashi, H. O. Hamaguchi and M. Watanabe, J. Phys. Chem. C, 2007, 111, 1541–1548 CAS.
- D. A. G. Bruggeman, Ann. Phys., 1935, 24, 636–664 CrossRef CAS.
- D. S. Mclachlan, J. Phys. C: Solid State Phys., 1987, 20, 865–877 CrossRef.
- C. W. Nan and D. M. Smith, Mater. Sci. Eng., B, 1991, 10, 99–106 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21378h |
| This journal is © The Royal Society of Chemistry 2012 |
