Synthesis of monolithic deep-ultraviolet-transparent polysilsesquioxane glasses from organotrimethoxysilane–water binary system
Koichi
Kajihara
*,
Arata
Sakuragi
,
Yuta
Igarashi
and
Kiyoshi
Kanamura
Department of Applied Chemistry, Graduate School of Urban Environmental Sciences, Tokyo Metropolitan University, 1-1 Minami-Osawa, Hachioji, 192-0397, Japan. E-mail: kkaji@tmu.ac.jp
First published on 3rd September 2012
Abstract
A facile sol–gel-based method for the formation of monolithic polysilsesquioxane glasses from acid-catalyzed organotrimethoxysilane–water binary mixtures, free from alcohols and organic solvents, has been developed.
Organically modified silica1,2 is an important class of organic–inorganic hybrids because the organic and inorganic parts are tightly bound through strong Si–C bonds. The building blocks of organically modified silicas include siloxys (R3SiO1/2), siloxanes (R2SiO2/2), and silsesquioxanes (SQs, RSiO3/2), where R denotes an organic functional group.3,4 Because of the structural variety, many different types of organically modified silicas have been developed. Dense transparent glassy monolithic organically modified silicas, such as polydimethylsiloxane (PDMS) resins, have usually been prepared using the siloxane units as the main components.5,6 The formation of dense glassy monoliths and films has also been explored with SQ-rich compositions to increase the silica content and develop the Si–O–Si network. For example, uniform polysilsesquioxane (PSQ) films have been prepared by thermal fusion of PSQ particles modified with phenyl7 and benzyl8 groups. Transparent monoliths have been formed by melting phenyl-modified gels containing both the siloxane and SQ units.9,10 Such gels, which soften at relatively low temperatures, around 100–200 °C , are often termed “melting gels”,11 and have attracted considerable attention as a new class of glassy material. However, monolithic dense glasses consisting solely of SQ units have yet to be realized, probably because PSQ gels are rigid as a result of the developed Si–O–Si network, and difficult to shape into a monolithic form by thermosoftening. In addition, the preparation methods for dense PSQ-based glasses need to be improved. Apart from the preparation of liquid PSQs from an acid-catalyzed toluene-based system,12 the reactions reported so far have been conducted under basic conditions using complicated multistep processes. Filtration11 and centrifugation7,8 of the precursor gels may lead to loss of the silica component. Alcohols9–11,13 and organic solvents,9,12 which are not essential components in sol–gel-based reactions,14,15 are frequently used as cosolvents to homogenize the reaction mixture and to control the reactions.
Another area of recent interest is glassy materials transparent to deep-ultraviolet (DUV, ≲300 nm) light, because of their increased use in sensing, sanitization, lighting, and processing. Most organic polymers and organic–inorganic hybrids for optical applications are not transparent to DUV light due to the optical absorption by functional groups containing unsaturated bonds, such as aryl and carbonyl groups. PDMS-based resins are also usually not DUV transparent, despite the good transparency of the main siloxane chain, because crosslinking functional groups, catalysts, and other minor additives absorb DUV light.
In this Communication, we present a simple sol–gel-based synthetic method for monolithic transparent PSQ glasses from organotrimethoxysilanes, without using alcohols, organic solvents, or other additives. When ethyltrimethoxysilane is used as the silicon source, PSQ glasses with good DUV transparency are realized.
Experimental procedure: methyltrimethoxysilane, ethyltrimethoxysilane, and phenyltrimethoxysilane were used as silica sources. A dilute aqueous solution of nitric acid was added at once to each alkoxide at an alkoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 mole ratio of 1
HNO3 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.002 and stirred for 3 h in a sealed container. The solution was aged for 1 d at 60 °C. Then the container was opened, the top layer of the biphasic solution was discarded, and the bottom layer was dried in vacuum for 24 h at 60 °C. The dried samples were heated in N2 or a vacuum to obtain glasses. The aged solutions and the dried samples were characterized using 1H NMR (JNM-ECS300, JEOL). The densities of the glass samples was measured using a pycnometer. Optical absorption spectra were measured using a conventional spectrometer (U-4100, Hitachi).
0.002 and stirred for 3 h in a sealed container. The solution was aged for 1 d at 60 °C. Then the container was opened, the top layer of the biphasic solution was discarded, and the bottom layer was dried in vacuum for 24 h at 60 °C. The dried samples were heated in N2 or a vacuum to obtain glasses. The aged solutions and the dried samples were characterized using 1H NMR (JNM-ECS300, JEOL). The densities of the glass samples was measured using a pycnometer. Optical absorption spectra were measured using a conventional spectrometer (U-4100, Hitachi).
Solutions obtained by mixing an organotrimethoxysilane with dilute aqueous nitric acid were initially clear and transparent. In the ethyl- and phenyl-modified systems, the solutions underwent liquid–liquid phase separation during aging at 60 °C. An example of the biphasic liquid, prepared from the ethyl-modified system, is shown in Fig. 1. Fig. 2 shows 1H NMR spectra of the top and bottom layers of the ethyl- and phenyl-modified systems after aging. The free methanol to R–Si group (R = Et or Ph) molar ratio in the top and bottom layers, calculated from the integration ratio of the methyl protons in methanol (peak e, δ ⋍ 3.45) and protons in the R–Si groups assuming that the R–Si bonds are intact, were ∼7.8 and ∼1.0 for the ethyl-modified system, and ∼13.6 and ∼1.1 for the phenyl-modified system. Total OH groups (OH groups in methanol, residual water, and PSQ) to R–Si group molar ratio in top and bottom layers were ∼11.2 and ∼1.3 for the ethyl-modified system, and ∼18.5 and ∼1.3 for the phenyl-modified system. Thus, the main components of the top layers were methanol and water, whereas the bottom layers consisted mainly of methanol and PSQ. These data confirm that the liquid–liquid phase separation is caused by the exsolution of PSQ, which is expected to be hydrophobic because of the alkyl or phenyl moieties, from the hydrophilic solvent mixture in the top layer. The slight upfield shift of the methyl proton of methanol in the bottom layer of the phenyl-modified system may be the result of electron donation from the phenyl groups to the methyl groups through the C–H/π interactions.16,17 In the methyl-modified system, in contrast, an opaque gel was obtained, suggesting that gelation of the PSQ component occurs in parallel with the phase separation into PSQ-rich and methanol-rich phases.
 | ||
| Fig. 1 Photograph of a biphasic solution obtained from the ethyl-modified system after aging. | ||
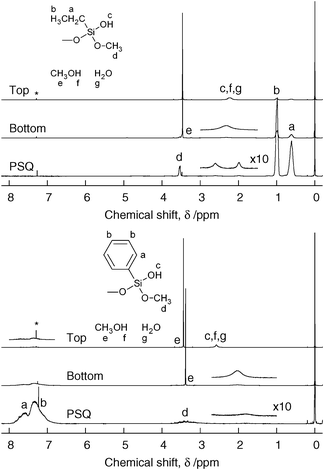 | ||
| Fig. 2 1H NMR spectra of top and bottom layers of the aged solution, and vacuum-dried PSQ (300 MHz, CDCl3): (top) ethyl-modified system, (bottom) phenyl-modified system. The asterisk at ∼7.3 ppm indicates the solvent residual peak. | ||
The top layer of the aged solution was removed by extraction, and the bottom layer was dried in a vacuum. Although this procedure leads to loss of some of PSQ dissolved in the top layer, this loss of PSQ can be avoided by simply drying the biphasic solution in a drying oven. In the phenyl-modified system, a transparent brittle solid was obtained. In contrast, the ethyl-modified sample did not solidify and remained viscous even after the vacuum drying. 1H NMR spectra of the dried samples are shown in Fig. 2. The sharp signal arising from residual methanol was very small in the ethyl-modified sample, and the mole fraction with respect to methoxy groups initially present in the precursor solution was ∼0.2%. This signal was hard to observe in the phenyl-modified sample. The signal at δ ⋍ 3.52 in the ethyl-modified sample and the broad signal at δ ⋍ 3.4 in the phenyl-modified sample are assigned to unhydrolyzed methoxy groups. These mole fractions were ∼3% for the ethyl-modified system and ∼5% for the phenyl-modified system. The broad signals at ∼2–3 are probably from OH groups. The mole fractions of OH protons with respect to that in the water initially added were ∼2% for the ethyl-modified system and ∼1% for the phenyl-modified system. The very small amount of residual solvent in the vacuum-dried PSQ samples indicates that the viscous flow of the ethyl-modified PSQ sample is intrinsic and not caused by remaining solvents. In the phenyl-modified system, in contrast, the π–π interactions between the phenyl groups probably hinder viscous flow of PSQ at room temperature.
Fig. 3 shows photographs and optical absorption spectra of monolithic ethyl- and phenyl-modified PSQ glasses. The ethyl-modified PSQ sample was cured in N2 for 4 d at 140 °C. The phenyl-modified PSQ powder was treated for 1 d at 100 °C , and this heating was performed in a vacuum to enhance defoaming during remelting of the powdered precursor. The densities (g cm−1) of the ethyl- and phenyl-modified glasses were ∼1.22 ± 0.03 and ∼1.32 ± 0.03, respectively. These values are comparable to the densities of conventional optical polymers [poly(methyl methacrylate) ∼1.2; amorphous polyolefin, ∼1.0–1.1; polycarbonate, ∼1.2]18 and silicone resins (∼0.9–1.1), but are much smaller than those of inorganic glasses (silica glass, ∼2.2; soda-lime glass, ∼2.5)19 and amorphous fluoropolymers (∼2).18 The ethyl-modified glass showed a good DUV transparency because of the absence of the functional groups that absorb DUV light. The UV absorption edge was located at ∼210 nm, showing a good DUV transparency compared with that of conventional optical polymers (≳260 nm),20 apart from amorphous fluoropolymers (∼150–170 nm).21 In the phenyl-modified glass, in contrast, the UV absorption edge was observed at ∼290 nm, and it is determined by the optical absorption of the phenyl groups.
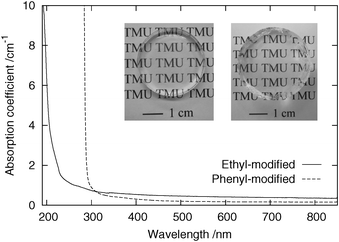 | ||
| Fig. 3 Optical absorption spectra of ethyl- and phenyl-modified PSQ glasses. Photographs of these samples (left, ethyl-modified sample, thickness ∼3.6 mm; right, phenyl-modified sample, thickness ∼8.5 mm) are also shown. | ||
In conclusion, polysilsesquioxane (PSQ) glasses were prepared in a simple manner from organotrimethoxysilane–water binary systems without using cosolvents such as alcohols and organic solvents. The liquid–liquid phase separation into PSQ-rich and methanol-rich phases during aging enables the recovery of methanol generated by the hydrolysis using simple extraction. The high silica content may be beneficial in increasing the strength and stability of PSQ glasses, and the low density makes them attractive for weight-saving in optical device applications. The ethyl-modified PSQ glass is transparent in the deep-ultraviolet spectral region. This method is probably applicable to the preparation of PSQs and their glasses with various different functional groups, and provides an opportunity for the development of PSQ-based functional materials.
This work was partially supported by the Nippon Sheet Glass Foundation for Materials Science and Engineering, and a Grant-in-Aid for Scientific Research (B) (No. 24350109) from the Japan Society for the Promotion of Science (JSPS).
References
- H. Schmidt, J. Non-Cryst. Solids, 1985, 73, 681 CrossRef CAS.
- J. Wen and G. L. Wilkes, Chem. Mater., 1996, 8, 1667 CrossRef CAS.
- R. H. Baney, M. Itoh, A. Sakakibara and T. Suzuki, Chem. Rev., 1995, 95, 1409 CrossRef CAS.
- Y. Abe and T. Gunji, Prog. Polym. Sci., 2004, 29, 149 CrossRef CAS.
- J. D. Mackenzie, J. Sol-Gel Sci. Technol., 1994, 2, 81 CrossRef CAS.
- C. Sanchez, B. Julián, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559 RSC.
- K. Katagiri, K. Hasegawa, A. Matsuda, M. Tatsumisago and T. Minami, J. Am. Ceram. Soc., 1998, 81, 2501 CrossRef CAS.
- A. Matsuda, T. Sasaki, K. Hasegawa, M. Tatsumisago and T. Minami, J. Am. Ceram. Soc., 2001, 84, 775 CrossRef CAS.
- H. Masai, M. Takahashi, Y. Tokuda and T. Yoko, J. Ceram. Soc. Jpn., 2005, 113, 259 CrossRef CAS.
- H. Masai, M. Takahashi, Y. Tokuda and T. Yoko, J. Mater. Res., 2005, 20, 1234 CrossRef CAS.
- L. C. Klein and A. Jitianu, J. Sol-Gel Sci. Technol., 2010, 55, 86 CrossRef CAS.
- K. Matsukawa, T. Fukuda, S. Watase and H. Goda, J. Photopolym. Sci. Technol., 2010, 23, 115 CrossRef CAS.
- H. Kakiuchida, M. Takahashi, Y. Tokuda, H. Masai, M. Kuniyoshi and T. Yoko, J. Phys. Chem. B, 2006, 110, 7321 CrossRef CAS.
- D. Avnir and V. R. Kaufman, J. Non-Cryst. Solids, 1987, 92, 180 CrossRef CAS.
- K. Kajihara, M. Hirano and H. Hosono, Chem. Commun., 2009, 2580 RSC.
- R. Ehama, M. Tsushima, T. Yuzuri, H. Suezawa, K. Sakakibara and M. Hirota, Bull. Chem. Soc. Jpn., 1993, 66, 814 CrossRef CAS.
- J. Ran and M. W. Wong, J. Phys. Chem. A, 2006, 110, 9702 CrossRef CAS.
- M. J. Weber, Handbook of Optical Materials, Laser and Optical Science and Technology Series, CRC Press, Boca Raton, 2003 Search PubMed.
- R. H. Doremus, Glass Science, 2nd edn, John Wiley & Sons, New York, 1994 Search PubMed.
- P. S. Nunes, P. D. Ohlsson, O. Ordeig and J. P. Kutter, Microfluid. Nanofluid., 1936, 4, 161 Search PubMed.
- R. H. French, R. C. Wheland, W. Qiu, M. F. Lemon, E. Zhang, J. Gordon, V. A. Petrov, V. F. Cherstkov and N. I. Delaygin, J. Fluorine Chem., 2003, 122, 63 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
