Formation of FexOy hollow nanospheres inside cage type mesoporous materials: a nanocasting pathway†
Hongxiao
Jin
a,
Chiya
Wang
a,
Bo
Hong
*a,
Langsheng
Ling
b,
Xiaojian
Gu
a,
Dingfeng
Jin
a,
Xiaoling
Peng
a,
Xinqing
Wang
a and
Hongliang
Ge
a
aZhejiang Province Key Laboratory of Magnetism, College of Materials Science and Engineering, China Jiliang University, Hangzhou, 310018, People's Republic of China. E-mail: Bohong@cjlu.edu.cn; bongbo@mail.ustc.edu.cn.; Fax: +86-571-8687-5600; Tel: +86-571-8687-5600
bHigh Magnetic Field Laboratory, Chinese Academy of Sciences, Hefei, 230031, People's Republic of China
First published on 9th October 2012
Abstract
FexOy hollow nanospheres were synthesized inside cage type mesoporous silica LP-FDU-12 through nanocasting. The sample was reduced to Fe3O4 and then further oxidized to γ-Fe2O3 with little changes in morphology and size. The Verwey transition is observed in the Fe3O4 hollow nanosphere system for the first time with magnetic measurement.
FexOy hollow structures have attracted a great deal of attention due to their unique physical and chemical properties.1 Based on the particle size, the hollow structures can be divided as follows: a particle size smaller than 30 nm and a particle size larger than 100 nm.2 The FexOy hollow structures of small size are potentially useful in magnetic resonance imaging contrast agents, drug-delivery vehicles, and separation of biomolecules.3 These smaller FexOy hollow structures are typically produced by template-free methods,4 including oxidation of Fe nanoparticles,5 gas phase oxidation6 and electron-beam irradiation of Fe nanoparticles.7 Another method for the formation of hollow structures is a templating approach,2,8 even though it offers important advantages such as narrowing the size distribution of the products, the early synthesis of the hollow structures through either soft or hard template methods often limits the particle size to within a few hundred nanometers.9 Therefore, it is still highly desirable to develop templating methods for the synthesis of hollow structures with a particle size smaller than 30 nm.
Since the first report on the nanocasting formation of the mesoporous carbon material from mesoporous silica,10 nanocasting has become a powerful tool for the synthesis of advanced functional materials.11 By using sacrificial templates, the replica structures of the template may be cast. The shape, size, and structure of the replica, which are “inherited” from the mother templates, can be systematically engineered by a rational choice of templates. To obtain really good replicas of the templates, it is of crucial importance to ensure that the pores of the templates are relatively well filled. However, interestingly, by varying the filling degree of the precursor in the void of a mesoporous material, the structure of the resulting replica can be varied. If SBA-15 with a 2D (p6![[m with combining macron]](https://www.rsc.org/images/entities/i_char_006d_0304.gif) m) hexagonal pore system is only coated by the carbon precursor instead of completely filling it, an array of hollow carbon tubes is obtained.12 If KIT-6 with a 3D bicontinuous cubic (Ia
m) hexagonal pore system is only coated by the carbon precursor instead of completely filling it, an array of hollow carbon tubes is obtained.12 If KIT-6 with a 3D bicontinuous cubic (Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d) pore system is partially coated by the carbon precursor, tube-like mesoporous carbons the same as the parent silica, were synthesized.13 Moreover, if KIT-6 is partially filled by the NiO precursor, mesoporous NiO would grow only on one of the two sets of pores.14 As reported in this communication, if FDU-12 with a 3D cubic (Im
d) pore system is partially coated by the carbon precursor, tube-like mesoporous carbons the same as the parent silica, were synthesized.13 Moreover, if KIT-6 is partially filled by the NiO precursor, mesoporous NiO would grow only on one of the two sets of pores.14 As reported in this communication, if FDU-12 with a 3D cubic (Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) spherical pore structure is partially filled with the FexOy precusor, FexOy hollow nanospheres are obtained. Despite its simple concept, no previous report was found on the successful formation of small hollow nanospheres (< 30 nm) inside a nanocage of highly ordered mesoporous materials.
m) spherical pore structure is partially filled with the FexOy precusor, FexOy hollow nanospheres are obtained. Despite its simple concept, no previous report was found on the successful formation of small hollow nanospheres (< 30 nm) inside a nanocage of highly ordered mesoporous materials.
In a standard nanocasting procedure, the three main steps involved are: (1) the rational choice and synthesis of the template; (2) casting the precursors and then converting them to target molecules; (3) removal of the template. Herein, in order to obtain FexOy hollow nanospheres through nanocasting, firstly, the void of the template must be spherical and have micro- or mesopores on the wall. Secondly, the spherical hollow structure should be partially filled with the FexOy precursor, otherwise solid spheres would form. And finally, if the precursors have a strong interaction with the wall molecules of the template, the replication would be much more facile. In consideration of the above points, LP-FDU-12 when treated with H2SO4 shows a large BET surface (1007.5 m2 g−1) area and pore volume (0.790 cm3 g−1), therefore it was selected as a hard template for the synthesis (Table S1 and Fig. S1, ESI†).15
Scheme 1 illustrates the procedure for generating FexOy hollow nanospheres. Fe(NO3)3•9H2O as the iron source was dissolved in ethanol, followed by the addition of the LP-FDU-12 mesoporous silica template. The mixture was stirred at room temperature until a nearly dry powder had been obtained. The sample was then heated slowly and calcined to pyrolyse the nitrate. The resulting sample was treated with a hot NaOH solution to remove the silica template, followed by washing with water and ethanol and then drying (see ESI† for more details). We first examined the morphology of the obtained FexOy hollow nanospheres after NaOH etching with field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). It can be clearly seen from the FESEM image (Fig. 1a) that the FexOy hollow nanospheres are very uniform and assemble into micro-particles. Fig. 1b and 1c show TEM images of the sample with an overall diameter of about 16.3 nm, which is comparable with the FESEM result, and the shell in the hollow structure is around 3.4 nm thick. Through examination of several single hollow nanoparticles with high-resolution TEM (HRTEM), we concluded that FexOy was a poor crystallization because the microcrystals were hardly detected (Fig. S2, ESI†). This is consistent with the XRD characterization where the patterns showed only some weak XRD peaks (Fig. S3b, ESI†). The nitrogen adsorption–desorption isotherms of the sample are type IV with a H1 hysteresis loop according to the IUPAC classification, indicating the mesoporous structure (Fig. S3a, ESI†). The specific BET surface area, pore volume, and mean pore diameters of the sample were measured to be 107.9 m2 g−1, 0.248 m3 g−1 and 13.5 nm, respectively (Table S1, ESI†).
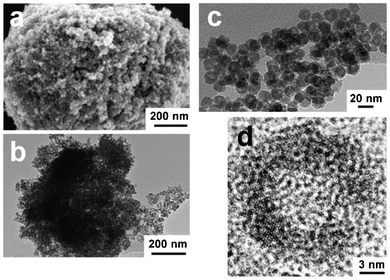 | ||
| Fig. 1 FESEM and TEM images of FexOy hollow nanospheres: (a) FESEM image, (b, c) TEM images, (d) HRTEM image. | ||
 | ||
| Scheme 1 Schematic formation of FexOy hollow nanospheres inside LP-FDU-12 silica. | ||
Previously, we had used a similar template to synthesize a series of mesoporous Co3O4 that showed a much lower specific BET surface area but a larger sphere size.16 In that case, we casted the Co3O4 precursor twice to ensure the full filling of the pores in order to obtain the replica of the template. However, in the present work, we casted the FexOy precursor only once and with a much lower concentration. We presumed the mechanism was as follows. The precursor would partially fill the void of the template with a high dispersion (Scheme 1). The precursor coated the pore surface due to the strong interaction of the precursor with the silanol on the surface. The LP-FDU-12 silica is composed of larger spherical pores connected with smaller cylinder necks. During the drying, the fluids in the spherical pores would evaporate first, because the surface tension of the fluids in the spherical pores is smaller than it is in the cylinder necks, thus, transporting the precursor from cylinder necks into the larger spherical pores, where the concentration of the precursor solution would be larger. Under high temperatures, the decomposition of the precursor to small FexOy particles involved both micropores and mesopores on the pore surface. With further heating, the recrystallization and in situ ripening of FexOy occurred, and the FexOy particles in the small pores might have moved to neighbouring large pores to minimize the surface energy. On the other hand, the mesopore confinement effect limited the particles to grow bigger, and once the small FexOy particles in the micropores (which are connected to the mesopores) moved to the mesopores, single nanoparticles as large as the mesopores were formed. To verify this mechanism, the LP-FDU-12 and FexOy hybrid after coating and heating steps was characterized by TEM and nitrogen adsorption–desorption. The pore size of the hybrid was measured to be 14.0 nm and 13.0 nm by nitrogen adsorption–desorption (Fig. S4c and S4d, ESI†) and TEM (Fig. S4b, ESI†), respectively, which is smaller than the original LP-FDU-12 (Table S1 and Fig. S4a, ESI†) template. The shrinkage of the pore size is due to the coating of the FexOy. We also assumed that the FexOy precursor might loosely disperse throughout the template on the pore surface after coating. However, it is difficult to directly observe the pore necks of the hybrid by TEM. Thus, it is still interesting to find the true origin of the formation of the FexOy hollow nanospheres inside a nanocage through further study.
The obtained FexOy was then reduced to Fe3O4 under H2/Ar (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95), and part of the resulting Fe3O4 was further oxidized to γ-Fe2O3 in air. The reduction and oxidation procedures were monitored by TG/DTA. An almost stoichiometric ratio of the reactants was transformed to products in both reactions (Fig. S5, ESI†). Fig. 2 shows the HRTEM images of Fe3O4 and γ-Fe2O3 hollow nanospheres. The reduction of α-Fe2O3 to Fe3O4 or oxidation of Fe3O4 to γ-Fe2O3 has caused little change to the morphology and size. The average sizes of these two hollow nanospheres were found to be about 16 nm and 15.8 nm for Fe3O4 and γ-Fe2O3 respectively (Table S1 and panel a, b in Fig. S6 and S7, ESI†). The void in the spheres became smaller after every step. The XRD patterns of the two samples suggested a higher crystallinity than the FexOy since more peaks were present (panel d in Fig. S6 and S7, ESI†). The nitrogen adsorption–desorption isotherms (panel c in Fig. S6 and S7, ESI†) of the Fe3O4 and γ-Fe2O3 hollow nanospheres were closely like that of the FexOy hollow nanospheres, which presented mesoporosity. The specific BET surface area, pore volumes, and mean pore diameters were measured to be 91.5 m2 g−1, 0.248 m3 g−1 and 14.7 nm for Fe3O4 and 88 m2 g−1, 0.222 m3 g−1 and 13.4 nm for γ-Fe2O3 respectively (Table S1, ESI†).
95), and part of the resulting Fe3O4 was further oxidized to γ-Fe2O3 in air. The reduction and oxidation procedures were monitored by TG/DTA. An almost stoichiometric ratio of the reactants was transformed to products in both reactions (Fig. S5, ESI†). Fig. 2 shows the HRTEM images of Fe3O4 and γ-Fe2O3 hollow nanospheres. The reduction of α-Fe2O3 to Fe3O4 or oxidation of Fe3O4 to γ-Fe2O3 has caused little change to the morphology and size. The average sizes of these two hollow nanospheres were found to be about 16 nm and 15.8 nm for Fe3O4 and γ-Fe2O3 respectively (Table S1 and panel a, b in Fig. S6 and S7, ESI†). The void in the spheres became smaller after every step. The XRD patterns of the two samples suggested a higher crystallinity than the FexOy since more peaks were present (panel d in Fig. S6 and S7, ESI†). The nitrogen adsorption–desorption isotherms (panel c in Fig. S6 and S7, ESI†) of the Fe3O4 and γ-Fe2O3 hollow nanospheres were closely like that of the FexOy hollow nanospheres, which presented mesoporosity. The specific BET surface area, pore volumes, and mean pore diameters were measured to be 91.5 m2 g−1, 0.248 m3 g−1 and 14.7 nm for Fe3O4 and 88 m2 g−1, 0.222 m3 g−1 and 13.4 nm for γ-Fe2O3 respectively (Table S1, ESI†).
 | ||
| Fig. 2 HRTEM images of hollow nanospheres: (a) Fe3O4, (b) γ-Fe2O3. | ||
Iron-based materials often have intriguing magnetic properties. Therefore, we examined the magnetic properties of the Fe3O4 and the γ-Fe2O3 hollow nanospheres on a SQUID magnetometer. The temperature dependence of the magnetization (M-T) in a weak applied field of 50 Oe is shown in Fig. 3a and 3b. For both samples, it can be seen that the value of the M decreases rapidly in the ZFC curve while there is not a big change in the FC curve with the temperature decreasing to the lower temperature. This immensely λ-type irreversibility between the ZFC and FC M-T curves should be due to the size effects and the existence of cluster-like spin glass.17 Specifically, there is an obvious kink in the FC curve at around 120 K of the Fe3O4 sample emerges (inset of Fig. 3a), which coincides with the well known Verwey (TV) transition of Fe3O4. Such a signature of the Verwey transition in Fe3O4 is not uncommon and has been reported before in other literature pertaining to Fe3O4.18 However, this is the first time this particular feature has been observed in the Fe3O4 hollow nanosphere system, which implies our Fe3O4 hollow sample has good crystallinity.
 | ||
| Fig. 3 The temperature dependence of the magnetization for hollow nanospheres (a) Fe3O4 and (b) γ-Fe2O3, and the magnetization as a function of the applied magnetic field for hollow nanospheres (c) Fe3O4 and (d) γ-Fe2O3. | ||
Fig. 3c and 3d show the magnetic hysteresis (M-H) loops combined with the expanded low-field hysteresis curves (insets) of the hollow nanospheres measured at 4 and 300 K, which indicate the magnetic properties, including saturation magnetization, Ms and the coercivity, Hc. The magnetic saturation is reached with the external field of about 6 T. The Ms values are about 59.45 emu g−1 at 4 K and 54.38 emu g−1 at 300 K for Fe3O4 hollow nanospheres, and 40.98 emu g−1 at 4 K and 36.32 emu g−1 at 300 K for γ-Fe2O3 hollow nanospheres, both of which are lower than those for corresponding bulk materials at 300 K (Ms = 92 emu g−1, Fe3O4, and 76 emu g−1, γ-Fe2O3) because of the spin disorder on the surface. The Hc values are about 871.3 Oe for Fe3O4 hollow nanospheres and 757.1 Oe for γ-Fe2O3 hollow nanospheres at 4 K respectively. The Hc is scarcely observed at 300 K for these two samples, indicating that the Fe3O4 and the γ-Fe2O3 hollow nanospheres should be in the superparamagnetic state at 300 K.
In summary, we have demonstrated a fabrication of FexOy hollow nanospheres through a nanocasting pathway. For the first time, highly ordered cage-type mesoporous silica was used as a hard template to generate hollow nanoparticles. Every nanocage could be viewed as a nanoreactor for the synthesis. The formation mechanism of the FexOy hollow nanospheres inside nanocages is still under investigation. The FexOy hollow nanospheres could be reduced to Fe3O4 and then further oxidised to γ-Fe2O3 with little changes in the morphology and size. The field-dependent magnetization measurements show that the samples are superparamagnetic and lack any hysteresis loops at 300 K. Specially, the Verwey transition is observed for the first time in the Fe3O4 hollow nanospheres system, which implies the material has good crystallinity. Due to the highly ordered and adjustable size of the nanoreactor, we expect that the “old” cage-type mesoporous materials will open new opportunities in the development of hollow nanospheres, especially smaller than 30 nm, with their adjustable sphere size and void space by rational control of the amount of precursors used and the pore size of the hard template.
We thank the National Natural Science Foundation of China (21001098 to H.X.J., 51172219 to H.L.G., and 21103154 to D.F.J.), the Natural Science Foundation of Zhejiang Province of China (Y4090475 to D.F.J., Y4100495 to H.X.J., and Z4090462 to H.L.G.), and Innovation Team Foundation of Science and Technology Department of Zhejiang Province of China (2010R50016 to H.X.J., B.H., W.X.Q., and H.L.G.). We also thank High Magnetic Field Laboratory, Chinese Academy of Sciences, for support.
References
- (a) C. N. R. Rao, H. Matte, R. Voggu and A. Govindaraj, Dalton Trans., 2012, 41, 5089–5120 RSC; (b) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS; (c) H. Goesmann and C. Feldmann, Angew. Chem., Int. Ed., 2010, 49, 1362–1395 CrossRef CAS; (d) R. Hao, R. J. Xing, Z. C. Xu, Y. L. Hou, S. Gao and S. H. Sun, Adv. Mater., 2010, 22, 2729–2742 CrossRef CAS; (e) W. Li, Y. Deng, Z. Wu, X. Qian, J. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Zhao, J. Am. Chem. Soc., 2011, 133, 15830–15833 CrossRef CAS; (f) W. Li, J. Yang, Z. Wu, J. Wang, B. Li, S. Feng, Y. Deng, F. Zhang and D. Zhao, J. Am. Chem. Soc., 2012, 134, 11864–11867 CrossRef CAS; (g) S. Sakurai, A. Namai, K. Hashimoto and S.-i. Ohkoshi, J. Am. Chem. Soc., 2009, 131, 18299–18303 CrossRef CAS.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS.
- (a) D. Ho, X. L. Sun and S. H. Sun, Acc. Chem. Res., 2011, 44, 875–882 CrossRef CAS; (b) K. Cheng and S. H. Sun, Nano Today, 2010, 5, 183–196 CrossRef CAS; (c) K. An and T. Hyeon, Nano Today, 2009, 4, 359–373 CrossRef CAS; (d) J. H. Gao, H. W. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef CAS; (e) B. H. Kim, N. Lee, H. Kim, K. An, Y. I. Park, Y. Choi, K. Shin, Y. Lee, S. G. Kwon, H. B. Na, J. G. Park, T. Y. Ahn, Y. W. Kim, W. K. Moon, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2011, 133, 12624–12631 CrossRef CAS.
- Q. Zhang, W. S. Wang, J. Goebl and Y. D. Yin, Nano Today, 2009, 4, 494–507 CrossRef CAS.
- S. Peng and S. H. Sun, Angew. Chem., Int. Ed., 2007, 46, 4155–4158 CrossRef CAS.
- C. M. Wang, D. R. Baer, L. E. Thomas, J. E. Amonette, J. Antony, Y. Qiang and G. Duscher, J. Appl. Phys., 2005, 98, 094308 CrossRef.
- A. H. Latham, M. J. Wilson, P. Schiffer and M. E. Williams, J. Am. Chem. Soc., 2006, 128, 12632–12633 CrossRef CAS.
- H. Groger, C. Kind, P. Leidinger, M. Roming and C. Feldmann, Materials, 2010, 3, 4355–4386 CrossRef.
- (a) S. J. Ding, J. S. Chen, G. G. Qi, X. N. Duan, Z. Y. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21–23 CrossRef CAS; (b) Y. Piao, J. Kim, H. Bin Na, D. Kim, J. S. Baek, M. K. Ko, J. H. Lee, M. Shokouhimehr and T. Hyeon, Nat. Mater., 2008, 7, 242–247 CrossRef CAS; (c) M. Sasidharan, H. N. Luitel, N. Gunawardhana, M. Inoue, S. Yusa, T. Watari and K. Nakashima, Mater. Lett., 2012, 73, 4–7 CrossRef CAS.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743–7746 CrossRef CAS.
- A. H. Lu and F. Schuth, Adv. Mater., 2006, 18, 1793–1805 CrossRef CAS.
- (a) S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169–172 CrossRef CAS; (b) A. H. Lu, W. Schmidt, B. Spliethoff and F. Schuth, Adv. Mater., 2003, 15, 1602–1606 CrossRef CAS.
- F. Kleitz, S. H. Choi and R. Ryoo, Chem. Commun., 2003, 2136–2137 RSC.
- F. Jiao, A. H. Hill, A. Harrison, A. Berko, A. V. Chadwick and P. G. Bruce, J. Am. Chem. Soc., 2008, 130, 5262–5266 CrossRef CAS.
- (a) J. Fan, C. Z. Yu, J. Lei, Q. Zhang, T. C. Li, B. Tu, W. Z. Zhou and D. Y. Zhao, J. Am. Chem. Soc., 2005, 127, 10794–10795 CrossRef CAS; (b) C. M. Yang, B. Zibrowius, W. Schmidt and F. Schuth, Chem. Mater., 2004, 16, 2918–2925 CrossRef CAS.
- H. Jin, X. Gu, B. Hong, L. Lin, C. Wang, D. Jin, X. Peng, X. Wang and H. Ge, J. Phys. Chem. C, 2012, 116, 13374–13381 CAS.
- J. M. DeTeresa, M. R. Ibarra, P. A. Algarabel, C. Marquina, J. Blasco, J. Garcia and A. delMoral, J. Appl. Phys., 1997, 81, 5504–5504 CrossRef CAS.
- (a) G. F. Goya, T. S. Berquo, F. C. Fonseca and M. P. Morales, J. Appl. Phys., 2003, 94, 3520–3528 CrossRef CAS; (b) F. X. Redl, C. T. Black, G. C. Papaefthymiou, R. L. Sandstrom, M. Yin, H. Zeng, C. B. Murray and S. P. O'Brien, J. Am. Chem. Soc., 2004, 126, 14583–14599 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: See DOI: 10.1039/c2ra21493h |
| This journal is © The Royal Society of Chemistry 2012 |
