Enhanced solid-state electrochemiluminescence of Ru(bpy)32+ immobilized on a laponite gel-state network and its glucose biosensing application
Shou-Nian
Ding
*a,
Bu-Hong
Gao
a,
Dan
Shan
b,
Serge
Cosnier
c and
Yue-Ming
Sun
a
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China
bSchool of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
cDépartment de Chimie Moléculaire UMR-5250, ICMG FR-2607, CNRS Université Joseph Fourier, BP-53, 38041 Grenoble, France. E-mail: snding@seu.edu.cn (S.-N. Ding); Fax: +86- 25-52090621; Tel: +86- 25-52090621
First published on 29th August 2012
Abstract
A novel laponite network thin film (denoted as laponitegel-film) was prepared by casting a laponite suspension with salt onto the surface of a glassy carbon electrode (GCE) to incorporate Ru(bpy)32+. The resultant Ru(bpy)32+-laponitegel-film/GCE presented significantly enhanced electrochemiluminescence (ECL) behavior. Thus, a glucose ECL biosensor was developed with a good performance.
Electrochemiluminescence (ECL) is a kind of chemiluminescence (CL) produced by an electrochemical reaction, which has been proven as a highly sensitive and selective detection method with a wide dynamic linear range and has been extensively applied in bioassays and clinical analysis by virtue of the absence of exciting light and the electrochemical control.1,2 Due to its reversible electrochemical behavior, high luminescent quantum efficiency and various analytes detection, the tris(2,2′-bipyridyl) ruthenium(II) complex (Ru(bpy)32+) and its derivatives have been the most extensively utilized to develop solid-state ECL sensors.3 Compared to aqueous ECL, solid-state ECL possesses the following advantages: (1) saving the expensive luminescent reagent consumption; (2) simplifying the equipment and the corresponding operation; (3) improving the separation efficiency of samples from combined flow systems, such as capillary electrophoresis (CE), microchip CE and high performance liquid chromatography (HPLC).
Until now, many protocols have been used to immobilize Ru(bpy)32+ to develop solid-state ECL sensors. These methods can be summarized into two categories: (1) synthesizing Ru(bpy)32+ derivatives with a 2,2′-bipyridyl ligand substitute tethered with functional groups, such as –SH, –Si(OEt)3 and –pyrrole,4–6 to anchor the luminophores to the solid matrices; (2) incorporating Ru(bpy)32+ directly into solid matrices via electrostatic adsorption, such as Nafion, poly(acrylonitrile-co-acrylic acid) nanofiber mat (PAN-co-PAAnfm) and gold colloids,7–9 or by entrapment into inorganic sol–gels or sol–gel hybrids, such as SiO2, TiO2 and ZrO2.10–14 However, for the former method, the synthesis procedures are tedious and complex while for the latter, the corresponding solid-state ECL sensors show some common drawbacks, such as a low luminescent intensity and the leakage of the luminophore from the surfaces of the electrodes and thereafter, the poor stability. Thus, it is still necessary to find new matrices to immobilize Ru(bpy)32+ to enhance the ECL intensity and keep the luminescent reagent from leaking.
Lamellar clay materials show specific physicochemical properties, such as high chemical stability, benign biocompatibility, good adsorption property due to their appreciable surface area, special structural feature and unusual intercalation property.15 Layered clays are potential scaffolds for the fabrication of biosensors. The synthetic lamellar clay, laponite (Na0.7[(Si8Mg5.5Li0.3)O20(OH)4]), purchased from Rockwood Specialties Inc., is composed of disc-shaped nanoparticles with a diameter of 30 nm and layer thickness of 1 nm with a high cation exchange ability (monovalent cation exchange capacity: CEC = 0.74 mmol g−1).
An isomorphic substitution of magnesium by lithium atoms generates a negative charge on its surface, which is counterbalanced by the positive charge of the sodium ions present in the interlayer. In aqueous media, sodium ions dissociate which, in turn, leads to a net negative charge on its surface. The edge of the laponite particle is composed of hydrous oxide and its charge is less negative in basic media, while it is positive in acidic media. Aqueous laponite suspensions display a very rich phase behavior with respect to the concentrations of the salt and the laponite. Generally, in salt-free aqueous alkaline media, the net interaction between various laponite particles in a dilute colloids solution is repulsive in nature namely, the repulsive glass state. However, the addition of a salt to a laponite colloid increases the concentration of cations in the system, which leads to screening the negative charges on the laponite surfaces. Consequently, the van der Waals attraction between the nanoparticles will dominate over the electrostatic repulsion, thereby changing the state of the system from a repulsive glass to a gel with a huge macroscopic network.16
In previous work, we have investigated the host–guest interaction of Ru(bpy)32+ and laponite. The electrochemical and ECL behaviors of Ru(bpy)32+ in laponite are related to the host–guest and guest–guest interactions. The adsorption of Ru(bpy)32+ onto surfaces rather than intercalating into the layers of laponite would enhance the ECL. Meanwhile, the enhanced ECL also contributes to the guest–guest interactions to form a house-in-cards structure.17
In the present work, we report the fabrication and characterization of a laponite gel film (laponitegel-film) exhibiting a porous network via the addition of a salt (potassium chloride) to the laponite suspension. The resulting laponitegel-film was employed for the incorporation of the luminescent Ru(bpy)32+ complex and the electrochemiluminescent process (ECL) was examined in water in the presence of oxalate as a co-reactant. The possibility of exploiting the enhanced ECL properties of such a composite electrode as a transduction signal for biological processes was investigated. For this purpose, an ECL biosensor was elaborated via the immobilization of glucose oxidase (GOD) as a protein model onto the Ru(bpy)32+-laponitegel-film electrode and applied to the detection of glucose.
All electrochemical and ECL experiments were carried out in a conventional three-electrode cell with a glassy carbon electrode (GCE, diameter 3 mm), a Pt wire and a saturated calomel electrode (SCE) as the working electrode, counter electrode and reference electrode, respectively. The ECL intensities were measured with an MPI-ECL Analyzer (Xi'an Remax Electronic High-Tech Ltd) through the bottom of the quartz glass electrochemical cell, which was placed in front of the photomultiplier tube (PMT) biased at −500 V. Ru(bpy)32+-laponitenormal-film/GCE was prepared as follows: (1) 2 mg mL−1 of a laponite colloidal suspension was prepared by dispersing laponite in twice distilled water with stirring overnight; (2) a drop of colloidal laponite (6 μL) was spread on the surface of the GCE and dried in air; (3) the as-prepared laponitenormal-film/GCE electrode was immersed into 5 × 10−4 mol L−1 Ru(bpy)32+ for 4 h. Ru(bpy)32+-laponitegel-film/GCE was fabricated according to the following procedure: (1) 2 mg of laponite was dispersed in 1 mL 2.5 × 10−2 mol L−1 of a KCl solution with stirring overnight; (2) 6 μl of the laponite suspension with a salt solution was coated onto the surface of the GCE and dried in air. Then the prepared electrode was washed thoroughly with twice distilled water to remove the excess salt from the film; (3) the resulting composite electrode was immersed in a 5 × 10−4 mol mL−1 Ru(bpy)32+ solution for 4 h. GOD/Ru(bpy)32+-laponitegel-film/GCE was prepared by spreading 6 μL of GOD (1 mg mL−1) onto the surface of the Ru(bpy)32+-laponitegel-film/GCE and cross-linking the protein molecules in a saturated glutaraldehyde atmosphere for 15 min. The bio-electrode was kept at 4 °C prior to use.
Oxalate is commonly used as a co-reactant in combination with Ru(bpy)32+ for anodic ECL studies, especially for the development of glucose ECL biosensors based on GOD. Herein, the ECL behavior of Ru(bpy)32+-laponitenormal-film/GCE and Ru(bpy)32+-laponitegel-film/GCE were firstly investigated in a 0.1 mol mL−1 phosphate buffer solution (PBS, pH 7.0) containing 5 × 10−3 mol L−1 ammonium oxalate by cycling the potential from 0 to 1.2 V with a scan rate of 100 mV s−1. Fig. 1 presents the resulting ECL intensity–time profiles. Surprisingly, it appears that the ECL peak intensity related to the Ru(bpy)32+-laponitegel-film/GCE (5200 counts) is ten times higher than that of the Ru(bpy)32+-laponitenormal-film/GCE (450 counts).
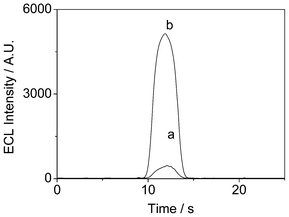 | ||
| Fig. 1 (a) The ECL emission of Ru(bpy)32+ immobilized in (a) a normal laponite film. (b) in a laponite-KCl composite film under cyclic voltammetry from 0 V to 1.2 V in 0.1 mol L−1 PBS containing 5 mmol L−1 C2O42−. Scan rate: 100 mV s−1. | ||
To get a better understanding and more information about this enhanced ECL performance, the ECL behaviors of the Ru(bpy)32+-laponitenormal-film and Ru(bpy)32+-laponitegel-film electrodes were recorded at a wider potential window, 0–1.5 V. Fig. 2 depicts the corresponding ECL intensity versus potential profiles during the potential cycling from 0 to 1.5 V in the presence of oxalate. As shown in Fig. 2A, two ECL peaks at 1.1 and 1.4 V were observed for the Ru(bpy)32+-laponitenormal-film electrode. According to previous research,17 the first ECL peak corresponds to the adsorbed Ru(bpy)32+ on the surface of laponite while the second is ascribed to the Ru(bpy)32+ intercalated between the laponite layers. In contrast, only one ECL peak at 1.1 V with a high intensity was observed for the Ru(bpy)32+-laponitegel-film electrode (Fig. 2B). This may be due to the porous network of laponitegel-film that presents a huge apparent surface area. Consequently, the majority of the Ru(bpy)32+ amount was immobilized by electrostatic interactions at the surfaces of the laponite network instead of being intercalated, thus explaining the higher ECL intensity at low potential.
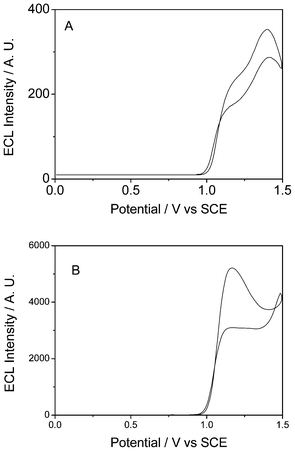 | ||
| Fig. 2 The ECL emission of Ru(bpy)32+ immobilized in a normal laponite film (A) and in a laponite gel network (B) in 0.1 mol L−1 PBS containing 5 mmol L−1 C2O42− under cyclic voltammetry from 0 V to 1.5 V. Scan rate: 100 mV s−1. | ||
The structure and morphology of the two laponite films were characterized by a tecnai F30 (FEI Co., USA) transmission electron microscopy (TEM). As shown in Fig. 3A, the normal laponite film was dense and compact. However, the TEM image of the salt (potassium chloride) decorated laponite film (Fig. 3B) exhibited a porous and loose structure, thus corroborating the preferential adsorption of Ru(bpy)32+ onto the surface of laponite. Furthermore, the porous structure facilitates the diffusion of the co-reagent into the film and its interaction with Ru(bpy)32+. Consequently, the Ru(bpy)32+ ECL intensity was much higher than that of Ru(bpy)32+ immobilized on a normal laponite film.
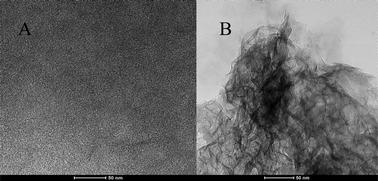 | ||
| Fig. 3 TEM images of normal laponite (A) and laponite gel (B) films. | ||
The ECL of the Ru(bpy)32+-laponitegel-film/GCE was quite stable. Fig. 4 shows the successive ECL emissions of a gel-laponite/Ru(bpy)32+/GCE under continuous CV from 0 to 1.2 V for 20 cycles. The maximum deviation amplitude in the ECL peak intensities is only 1.8% for 20 consecutive scanning cycles and the standard derivation of the ECL peak intensities is 0.9%. No obvious change in the ECL intensity was observed, illustrating the mechanical stability of the incorporated Ru(bpy)32+ within the laponite matrix.
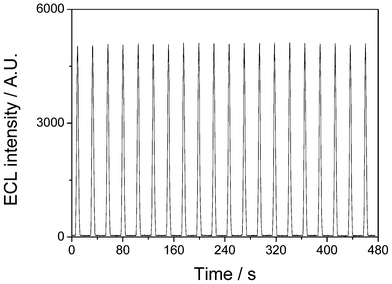 | ||
| Fig. 4 ECL emission of Ru(bpy)32+ immobilized in a laponite-gel film in 0.1 mol L−1 PBS containing 5 mmol L−1 C2O42− under continuous CV from 0 V to 1.2 V for 20 cycles. Scan rate: 100 mV s−1. | ||
Rotating disk electrode experiments (RDE) were also performed to compare the film permeability of the two inorganic coatings. The experiments were carried out at different rotation rates in the presence of hydroquinone as a redox probe (2 × 10−3 mol L−1) in 0.1 mol L−1 PBS (pH 7.0). Rotating disk voltammograms were also recorded with a bare GCE as a control. Fig. 5 reveals that the steady-state limiting currents (ilim) were estimated to be 2.17, 1.93 and 1.31 mA cm−2 for the bare GCE, laponitegel-film/ GCE and laponitenormal-film/GCE, respectively.
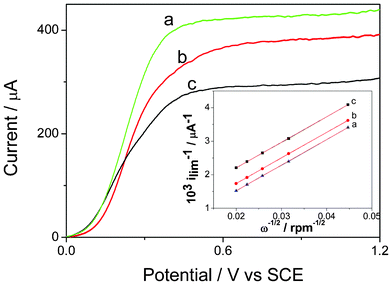 | ||
| Fig. 5 Rotating-disk electrode voltammograms of (a) naked GCE (b) the gel laponite/GCE and (c) the laponite/GCE in PBS (pH 7.0) containing 2 mmol L−1 hydroquinone (υ = 10 mVs−1, ω = 1000 rpm). Inset: Koutecky-Levich plots for (a) naked GCE, (b) the gel laponite network/GCE and (c) the laponite/GCE. | ||
Through the ilim of the three different electrodes, it is easy to see that laponitenormal-film/GCE has a higher diffusion resistance to the substrate than the laponitegel-film/GCE. The permeability (Pm) of the film was calculated by eqn (1)–(4) and reported by Gough and Leypoldt as the variation of the steady-state limiting current ilim with the mass transport for a rotating disk electrode coated with an electro-inactive membrane.18
| 1/ilim = 1/is+1/im | (1) |
| is = 0.62nFC0Ds2/3v−1/6ω1/2 | (2) |
| im = nFSC0KDm/δ = nFSC0Pm | (3) |
The terms Ds and Dm are the diffusion coefficients for the substrate in the bulk solution and in the membrane, respectively, v is the kinematic viscosity of the solution, ω the rotating rate of the RED, δ the thickness of the membrane, K the partition equilibrium constant of the substrate between the solution and membrane, S the electrode surface, n the number of exchanged electrons, F the Faraday constant and C0 the substrate concentration. Only the first term of eqn (1) is dependent upon the rotation rate of the RDE while the second reflects the permeability Pm, defined as Pm = KDm/δ cm s−1. As a consequence, the experimental data from RDE voltammograms was displayed in the form of Koutecky-Levich plots (as shown in the inset of Fig. 5). A plot of 1/ilimversus 1/ω1/2 thus exhibits a linear behavior with the same slope that was obtained for a bare electrode. The value of the positive intercept can reflect the permeability Pm of the membranes. The permeability value for the laponitegel film/GCE was calculated as 6.1 × 10−2 cm s−1, about 3.2 times greater than that of the laponitenormal film/GCE (1.9 × 10−2 cm s−1). The higher permeability of laponitegel film/Ru(bpy)32+/GCE leads to higher and more efficient mass transfer of the reactants.
Fig. 6 shows the ECL obtained for the GOD/Ru(bpy)32+-laponitegel-film/GCE in PBS with different glucose concentrations. The glucose solution was allowed to mutarotate at room temperature for 24 h before use. It is clearly shown that the ECL intensity in the presence of glucose was lower than that in the absence of glucose. In addition, the ECL intensity decreased gradually with an increasing concentration of glucose. Based on the experimental results, the calibration curve for glucose detection was plotted on a logarithmic axis to show the wide dynamic ranges. The linear range covered from 1.0 × 10−6 to 4.0 × 10−3 mol L−1 (R2 = 0.997) with a detection limit of 7.0 × 10−7 mol L−1 (S/N = 3).
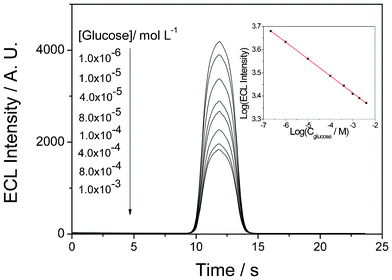 | ||
| Fig. 6 ECL profiles of the biosensor as a function of glucose concentration in PBS (PH 7.0) containing 5 mmol L−1 C2O42−. Inset: Corresponding calibration curve of glucose with the prepared ECL biosensor. | ||
The reason for detecting glucose was that H2O2, generated from the reaction catalyzed by GOD between glucose and oxygen, consumes ammonium oxalate. The depletion in oxalate concentration thus hindered the Ru(bpy)32+ ECL reaction. To support this point, a control experiment to show the effect of H2O2 on the ECL behavior of the Ru(bpy)32+-laponitegel-film/GCE has been performed. Fig. 7 depicts the ECL profiles of Ru(bpy)32+ immobilized in a laponite-gel film in 0.1 mol L−1 PBS (pH 7.0) containing 5 mmol L−1 C2O42− without (Original) and with 1 mM of glucose plus various H2O2 concentrations (0 μM, 1.0 μM, 10 μM, 100 μM and 1 mM), respectively. The addition of glucose has almost no effect on the ECL behavior, while the ECL intensities obviously decreased with increasing H2O2 concentrations.
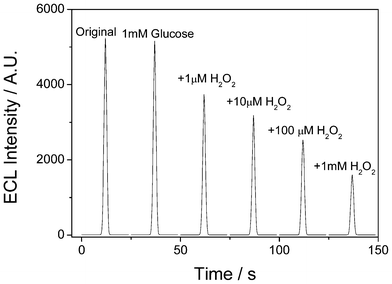 | ||
| Fig. 7 ECL profiles of Ru(bpy)32+ immobilized in a laponite-gel film in 0.1 mol L−1 PBS (pH 7.0) containing 5 mmol L−1 C2O42− without (Original) and with 1 mM glucose plus various H2O2 concentrations (0 μM, 1.0 μM, 10 μM, 100 μM and 1 mM), respectively under cyclic voltammetry from 0 V to 1.2 V. Scan rate: 100 mV s−1. | ||
In summary, laponite gel with a porous network structure-modified GCE electrode was easily prepared by the addition of potassium chloride and constitutes an attractive matrix for the immobilization of Ru(bpy)32+ by the electrostatic adsorption onto the surface of the laponite gel film. The resulting porous structure exhibits a good permeability for the diffusion of the co-reagent into the film, leading to an enhancement of the ECL process by a factor of ten compared to the conventional laponite film. Furthermore, GOD was used as a protein model to successfully fabricate ECL biosensors to detect glucose via the effect of H2O2 on oxalate. Such an approach may be useful for the development of ECL immunosensors, the immunoreaction hindering the permeation of the oxalate co-reactant or with a labeling step of the antibody target by a secondary antibody conjugated with GOD.
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation (No. 20905011, 21173042, 21010102071), the Natural Science Foundation of Jiangsu (No. BK2010396, BK2011589), the Scientific Research Foundation for the returned overseas Chinese scholars, MOE, the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University and the Southeast University Creative Foundation (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207
207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040
040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501).
501).
References
- L. Z. Hu and G. B. Xu, Chem. Soc. Rev., 2010, 39, 3275–3304 RSC.
- X. M. Chen, B. Y. Su, X. H. Song, Q. A. Chen, X. Chen and X. R. Wang, TrAC, Trends Anal. Chem., 2011, 30, 665–676 CrossRef CAS.
- H. Wei and E. Wang, TrAC, Trends Anal. Chem., 2008, 27, 447–459 CrossRef CAS.
- Y. S. Obeng and A. J. Bard, Langmuir, 1991, 7, 195–201 CrossRef CAS.
- G. M. Greenway, A. Greenwood, P. Watts and C. Wiles, Chem. Commun., 2006, 85–87 RSC.
- S. N. Ding, S. Cosnier, D. Shan, Y. M. Sun and Y. Wang, Electrochem. Commun., 2010, 12, 905–907 CrossRef CAS.
- N. Oyama and F. C. Anson, Anal. Chem., 1980, 52, 1192–1198 CrossRef CAS.
- D. Shan, B. Qian, S. N. Ding, W. Zhu, S. Cosnier and H. G. Xue, Anal. Chem., 2010, 82, 5892–5896 CrossRef CAS.
- X. P. Sun, Y. Du, S. J. Dong and E. Wang, Anal. Chem., 2005, 77, 8166–8169 CrossRef CAS.
- M. M. Collinson, B. Novak, S. A. Martin and J. S. Taussig, Anal. Chem., 2000, 72, 2914–2918 CrossRef CAS.
- H. N. Choi, S. H. Cho and W. Y. Lee, Anal. Chem., 2003, 75, 4250–4256 CrossRef CAS.
- S. N. Ding, J. J. Xu and H. Y. Chen, Electrophoresis, 2005, 26, 1737–1744 CrossRef CAS.
- S. N. Ding, J. J. Xu and H. Y. Chen, Electroanalysis, 2005, 17, 1517–1522 CrossRef CAS.
- S. N. Ding, J. J. Xu, W. J. Zhang and H. Y. Chen, Talanta, 2006, 70, 572–577 CrossRef CAS.
- D. Shan, C. Mousty and S. Cosnier, Anal. Chem., 2004, 76, 178–183 CrossRef CAS.
- Y. M. Joshi, G. R. Reddy, A. L. Kulkarni, N. Kumar and R. P. Chhabra, Proc. R. Soc. London, Ser. A, 2008, 464, 469–489 CrossRef CAS.
- D. Shan, S. N. Ding, J. J. Xu, W. Zhu, T. Zhang, H. Y. Chen and S. Cosnier, Electrochem. Commun., 2010, 12, 227–230 CrossRef CAS.
- A. Gough and J. K. Leypold, Anal. Chem., 1979, 51, 439–443 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
