Controlled synthesis of Co3O4 spinel with Co(acac)3 as precursor†
Patrick Mountapmbeme
Kouotou
,
Zhen-Yu
Tian
*,
Udo
Mundloch
,
Naoufal
Bahlawane‡
and
Katharina
Kohse-Höinghaus
Department of Chemistry, Bielefeld University, Universitätsstraße 25, D-33615 Bielefeld, Germany. E-mail: zhenyu.tian@uni-bielefeld.de; Fax: +49-521 1066027; Tel: +49-521 1062199
First published on 3rd September 2012
Abstract
Cobalt(III) acetylacetonate (Co(acac)3) was used as a precursor to grow pure Co3O4 with pulsed-spray evaporation chemical vapor deposition (PSE-CVD). The effect of solvent and substrate temperature on the growth kinetics and morphology of the films was investigated. The obtained spinel exhibited good catalytic performance.
Among transition metal oxides, Co3O4 exhibits interesting electrical, magnetic, optical and catalytic properties.1 In the last decade, the catalytic activity of Co3O4 and Co-based spinels has substantially increased the interest towards their technological application.2 The promising ability of Co3O4 to exchange its lattice oxygen with the atmosphere sustains its high potential application in air pollution control via the oxidative abatement of CO3 and organic pollutants from exhaust streams.4
Several methods have been reported previously to obtain Co3O4 thin films, including sol–gel,5 spray pyrolysis,6 atomic layer deposition,7 sputtering8 and chemical vapor deposition (CVD).9–11 Among these techniques, pulsed-spray evaporation CVD (PSE-CVD) is considered a promising method to prepare pure films due to its low cost, simplicity, high throughput and easy control of the thickness and morphology of the samples. Particularly, PSE-CVD is suitable for precursors with limited thermal stability and well adapted for systematic synthesis of single-phase spinel or perovskite oxides with tailored composition on various relevant substrates.
Most efforts to grow Co3O4 spinel by CVD involved several precursors (Table S1, ESI†). Metal–organic compounds (MOC) used as precursors are either commercially available or can be easily prepared in the laboratory. Of all the MOC, cobalt β-diketonates serve as non-toxic (environmentally friendly), volatile and inexpensive precursors. In the family of β-diketonates, cobalt acetylacetonates are the easiest to prepare, requiring no special atmosphere or environment.12 Recent investigations reported the use of cobalt(II) acetylacetonate (Co(acac)2) for the synthesis of Co3O4.13,14 However, this precursor shows instability in several solvents, and the selection of alcohol presents a particularly complicated effect on the formed cobalt phase.13 In contrast to other solvents, alcohols exhibit certain reactivity with cobalt acetylacetonate under deposition conditions. This reactivity could lead to the formation of carbide phases at low temperature in the absence of oxygen.15 Therefore, an alternative may be needed.
The present work is focused on the controlled synthesis of Co3O4 spinel by PSE-CVD using cobalt(III) acetylacetonate (Co(acac)3) as precursor and the investigation of the effect of solvent and substrate temperature (Ts) on the growth kinetics and film morphology. Moreover, the catalytic performance of the sample obtained with tetrahydrofuran (THF) as the solvent was tested in terms of total oxidation of CO and propene (C3H6) in a flow reactor.
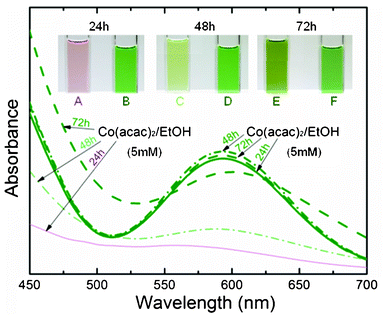
Special attention has been paid to the effect of aging on the stability and the ultraviolet-visible (UV-vis) absorption spectrum of the ethanol (EtOH) solution containing Co(acac)2 and Co(acac)3, respectively. The solutions were stored in a closed atmosphere for several days and the results are shown in Fig. 1. For Co(acac)2/EtOH solution (A, C and E), the pink color transforms gradually into dark green, and a broad optical absorption band peaking at 590 nm is observed progressively after three days. However, neither color nor UV-vis spectra are changed for Co(acac)3/EtOH solution (B, D and F). The absorption peak is characteristic of an octahedral Co(III) species,16 which indicates that Co(acac)3 is more stable than Co(acac)2 and thus more suitable for the CVD processes with liquid-based feedstocks. Moreover, Co(acac)3 was reported to be more thermally stable than Co(acac)2.17 Even though studies on the development of CVD precursors (CoIII β-diketonates) have been reported,18,19 systematic and extensive investigation of the Co3O4 thin film synthesis starting from Co(acac)3 in several solvents system remains desirable. The Co(acac)3 precursor–solvent system was analyzed systematically with respect to the deposition results. The growth of cobalt oxide thin films was carried out in a cold-wall CVD reactor which is equipped with a home-built PSE unit for the precursor delivery. The description of the experimental setup can be found elsewhere.13 Further details of the experimental conditions are available (Table S2, ESI†).
The optimization of the Co3O4 growth was achieved using Co(acac)3 in EtOH as liquid feedstock. The thickness of the obtained films was estimated gravimetrically using a microbalance (Mettler ME30, digital resolution of 1 μg). The optimal growth rate, ∼1.6 nm min−1 at 20 mbar and a substrate temperature of 350 to 500 °C, was obtained using an O2 flow rate of 0.5 SLM (standard liter per minute). In order to determine the effect of the agent in the synthesis of pure Co3O4, different types of solvents were employed: EtOH (polar protic solvent), THF (polar aprotic solvent) and toluene (non polar solvent). The results indicate that the growth rate varies slightly depending on the solvents and a maximum value of ∼3 nm min−1 was obtained with THF. With the investigation of the gas phase at lower temperatures (160–250 °C) using molecular beam mass spectroscopy, the product pool is proven to be quite stable for a specific solvent with and without Co(acac)3, as shown in Figs. S1–S3, ESI†. However, the composition of the gas mixtures may evolve with oxidation reactions at higher temperatures (see Fig. S4, ESI†) and would vary the deposition process. The discussion of the detailed gas phase reaction is beyond of the current work and will be explained in a forthcoming study.
For characterization purposes, films were deposited on silicon, glass and stainless steel. The obtained films were subjected to XRD and SEM analyses, and the results are depicted in Fig. 2 and 3, respectively. As shown in Fig. 2, the films obtained at Ts = 350/450 °C present different XRD patterns depending on both the deposition temperature and the nature of the solvent. The positions of the diffraction peaks fit well with the cubic spinel-type structure of Co3O4 (JCPDS Nr. 74-1656). No peaks associated to other crystalline forms could be detected, indicating that the obtained samples are of high purity and crystallinity.
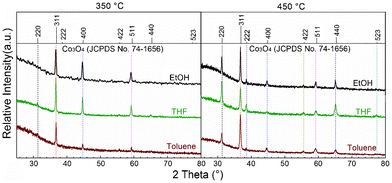 | ||
| Fig. 2 XRD Patterns of the investigated Co3O4 spinels. | ||
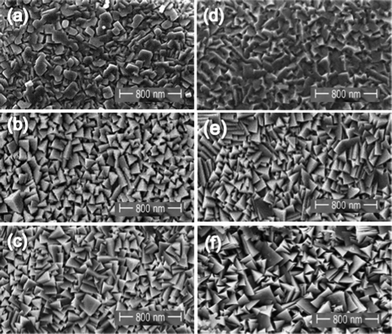 | ||
| Fig. 3 SEM micrographs of Co3O4 obtain respectively at 350 and 450 °C (a, d) with ethanol; (b, e) with toluene; (c, f) with THF. | ||
The effect of Ts on the growth of the film was evaluated. The absence of some reflection planes is observed at Ts = 350 °C. The films grown at high temperature seem closer to a polycrystalline structure. However, all peaks fit well with the reference at 450 °C and no enhanced orientation can be claimed. EtOH, toluene and THF lead to the growth of films starting at a temperature of 350 °C, and Co3O4 is the crystalline phase in the investigated Ts range (350–500 °C). A clear identification of the spinel layer by XRD was obtained for the grown films at 350/450 °C with a thickness of ∼300 nm over glass and silicon independent of the solvent. Increasing temperature from 350 to 450 °C, the particles size increases by a few nanometers whereas the lattice parameter remains quite constant (see Table 1).
| Solvents | T s (°C) | Growth (nm min−1) | a/Å | Particle size (nm) |
|---|---|---|---|---|
| EtOH | 350 | 1.55 | 8.10056 | 30.4 |
| 450 | 1.52 | 8.10306 | 40.0 | |
| Toluene | 350 | 1.91 | 8.08985 | 39.5 |
| 450 | 2.17 | 8.09642 | 42.2 | |
| THF | 350 | 2.16 | 8.09233 | 39.8 |
| 450 | 2.89 | 8.08985 | 59.0 |
The behavior of different solvents with different physical properties (Table S3, ESI†) on the growth and morphology of the Co3O4 thin films was also investigated. Compared to a relatively slow solubility in EtOH, Co(acac)3 was observed to dissolve rapidly in both THF and toluene. Prolonged use of the precursor in the EtOH system causes crystallization of the precursor, which clogs of the spray nozzle and consequently limits its efficiency in the deposition experiments.
Besides the general effect of temperature and solvent on the growth rate and composition, it is demonstrated that the morphology of the formed Co3O4 can also be influenced by the choice of the solvents. As displayed in Fig. 3, the morphology of the deposited films indicates compact and geometrical particles, which differ with Ts and solvents. The coverage of the surface is homogeneous with THF and toluene as solvents, consisting of connected micrometer-sized agglomerates, whereas films are less defined with EtOH. Films deposited at 350 and 450 °C using EtOH as solvent, were randomly oriented with poorly crystallized grains. When THF and toluene were employed, the obtained films are randomly oriented, well crystallized and present better defined facets. The Co3O4 crystallites showed trigonal pyramidal shape for films deposit at 350/450 °C with THF and toluene. In the explored temperature range, the increase of the growth rate as a function of the deposition temperature and the nature of the solvent can be explained in terms of variation of the surface diffusion with temperature and the co-adsorbed solvent. With EtOH at Ts = 350/450 °C, due to its reducing tendency (related to hydrogen from the alcohol function), the nucleation density becomes higher, giving rise to a fine-grained thin layer with low crystallinity. However, the surface diffusion was increased at the same Ts with THF/toluene, the chemical nature of which does not exhibit the reducing tendency. Consequently, the nucleation density was decreased, which could result in larger and well-crystallized particles. The temperature and the nature of the solvent dominate the film growth and affect its morphology. Various factors should be considered in understanding these complex processes, such as the solvent boiling point, vapour pressure, solubility, and polarity which have also considerable impact on the morphology of the final film.20
Although the mechanisms involved in the development of the film morphology and the resultant microstructure are still speculative, the roles played by the Ts and solvent on the film morphology have been demonstrated. Such an influence could be caused by the reaction kinetics affecting the competitive nucleation and growth rates. For example, the inhomogeneous nucleation and rapid growth rates combined with low solubility of EtOH are responsible for non-effective dissolution, which could result in the formation of the observed amorphous morphology. On the other hand, a more homogeneous nucleation and slower growth combined with an effective separation of the particles in THF and toluene could lead to the fine particulate morphology.
With the deposition process optimized and properties analyzed using Co(acac)3 as the precursor, it is interesting to compare catalytic performance with that of previous systems using Co3O4. In this work, no particular enhancement of the specific surface area and porosity can be noticed in all cases, which enables us to simplify the analysis of the catalytic activity. To assess the catalytic activity, oxidation of CO and C3H6 was performed over Co3O4 (12 mg, coated on mesh of stainless steel, SPW 40, 80 × 400) prepared using Co(acac)3/THF at 350 °C. The total flow rate was kept at 15 ml min−1 with 1% fuel/10% O2 dilute in Ar. A reflectron time-of-flight mass spectrometer (RTOFMS) was used for the analysis of the exhaust gas. The obtained Co3O4 catalysts exhibited excellent activity (see Fig. 4). Starting at around 230 °C, the total conversion of the investigated compounds to CO2 is obtained at around 380 °C for C3H6 and 350 °C for CO, which gives an obvious temperature shift relative to the non-coated mesh. This result shows that Co3O4 is very active for total oxidation of CO, in line with the literature16 (see Table S3 ESI†). The excellent catalytic activity towards the oxidation of CO is attributed to the abundance of active Co3+ cations and oxygen vacancies on film surface. According to the assumption of Xie et al.21 and Hu et al.,22 Co3+cations and oxygen vacancies acted as the active sites for CO oxidation and were key parameters governing the conversion rate of CO in the catalytic process. In fact, in the presence of CO and O2, CO can be adsorbed on the Co3+ sites of Co3O4 and react with the weakly bound surface oxygen species such as bridged Co3+–O2−–Co2+, leading to the production of CO2, and the resulting oxygen vacancy is subsequently filled by reaction with O2.23
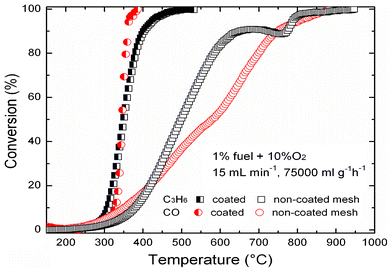 | ||
| Fig. 4 Conversion of C3H6 and CO over Co3O4. | ||
Below 200 °C, Co3O4 exhibits no effect on the catalytic oxidation of C3H6, but increasing the temperature, we obtained 90% conversion at 380 °C, which is in excellent agreement with our recent work,24 and presents competitive activity to that reported for noble metals (Table S3 ESI†).25,26
The catalytic activity of Co3O4 was explained in terms of formation of surface oxygen vacancies which are relevant to the C3H6 oxidation in the presence of gaseous oxygen. The surface lattice oxygen and adsorbed oxygen generally present on the transition metal oxides suggest that the C3H6 oxidation preferentially takes place on the surface lattice oxygen and adsorbed oxygen sites. Moreover, the presence of electrons in the neighbourhood of holes is necessary for the fission of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in alkenes.27
C bond in alkenes.27
In summary, Co(acac)3 in both toluene and THF have been found to be suitable combinations for the deposition of pure Co3O4 spinel by PSE-CVD at a total pressure of 20 mbar. The morphology and growth rate have been found to be dependent on the solvent and substrate temperature. The obtained Co3O4 exhibits promising catalytic performance for the complete conversion of C3H6 as well as CO to CO2, comparable to noble metals.
Dr Tian and Mr. Mountapmbeme Kouotou thank the Alexander von Humboldt foundation and the Deutscher Akademischer Austausch Dienst (DAAD) respectively for their research stay at Bielefeld University in Germany. The authors acknowledge additional support by the Bielefelder Nachwuchsfonds and Prof. Thomas Hellweg for access to the SEM.
References
- H. Liang, J. M. Raitano, L. Zhang and S.-W. Chan, Chem. Commun., 2009, 48, 7569 RSC.
- R. Bashyam and P. Zelenay, Nature, 2006, 443, 63 CrossRef CAS.
- Y. J. Mergler, J. Hoebink and B. E. Nieuwenhuys, J. Catal., 1997, 167, 305 CrossRef CAS.
- A. S. K. Sinha and V. Shankar, Chem. Eng. J., 1993, 52, 115 CrossRef CAS.
- K. J. Kim and Y. R. Park, Solid State Commun., 2003, 127, 25 CrossRef CAS.
- P. S. Patil, L. D. Kadam and C. D. Lokhande, Thin Solid Films, 1996, 272, 29 CrossRef CAS.
- A. Rautiainen, M. Lindblad, L. B. Backman and R. L. Puurunen, Phys. Chem. Chem. Phys., 2002, 4, 2466 RSC.
- H. Yamamoto, S. Tanaka and K. Hirao, J. Appl. Phys., 2003, 93, 4158 CrossRef CAS.
- C. Guyon, A. Barkallah, F. Rousseau, K. Giffard, D. Morvan and M. Tatoulian, Surf. Coat. Technol., 2011, 206, 1673 CrossRef CAS.
- N. Bahlawane, E. Fischer Rivera, K. Kohse-Höinghaus, A. Brechling and U. Kleineberg, Appl. Catal., B, 2004, 53, 245 CrossRef CAS.
- E. Fischer Rivera, B. Atakan and K. Kohse-Höinghaus, J. Phys. IV, 2001, 11, 629 CrossRef.
- T. T. Kodas, M. J. Hampden-Smith, The Chemistry of Metal CVD; VCH: Weinheim, Germany, 1994 Search PubMed.
- P. A. Premkumar, N. Bahlawane and K. Kohse-Höinghaus, Chem. Vap. Deposition, 2007, 13, 219 CrossRef CAS.
- Z. Y. Tian, N. Bahlawane, F. Qi and K. Kohse-Höinghaus, Catal. Commun., 2009, 11, 118 CrossRef CAS.
- P. A. Premkumar, A. Turchanin and N. Bahlawane, Chem. Mater., 2007, 19, 6206 CrossRef CAS.
- S. Tanase, E. Bouwman, J. Reedijk, W. L. Driessen, M. Ferbinteanu, M. Huber, A. M. Mills and A. L. Spek, Eur. J. Inorg. Chem., 2004, 1963 CrossRef CAS.
- B. Atakan and M.A. Siddiqi, Proc. Euro-CVD, 2005, 15, 229 Search PubMed.
- H. Lee, C. H. Lee, I. S. Oh and I. M. Lee, Bull. Korean Chem. Soc., 2010, 31, 891 CrossRef CAS.
- M. Burriel, G. Garcia, J. Santiso, A. N. Hansson, S. Linderoth and A. Figueras, Thin Solid Films, 2005, 473, 98 CrossRef CAS.
- L. M. Chen, Z. Hong, G. Li and Y. Yang, Adv. Mater., 2009, 21, 1434 CrossRef CAS.
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, Nature, 2009, 458, 746 CrossRef CAS.
- L. Hu, K. Sun, Q. Peng, B. Xu and Y. Li, Nano Res., 2010, 3, 363 CrossRef CAS.
- Y. Yu, T. Takei, H. Ohashi, H. He, X. Zhang and M. Haruta, J. Catal., 2009, 267, 121 CrossRef CAS.
- Z. Y. Tian, P. H. Tchoua Nganou, V. Vannier, K. Kohse-Höinghaus and N. Bahlawane, Appl. Catal., B, 2012, 117-#x2013;118, 125 Search PubMed.
- R. Zhang, H. Alamdari and S. Kaliaguine, Catal. Lett., 2007, 119, 108 CrossRef CAS.
- S. Ivanova, C. Petit and V. Pitchon, Gold Bull., 2006, 39, 3 CrossRef CAS.
- M. Anpo, I. Tanahashi and Y. Kubokawa, J. Chem. Soc., Faraday Trans. 1, 1982, 78, 2121 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: list of used cobalt precursor, experimental condition, solvent properties, comparison with other results, gas phase evolution of different solvents with and without Co(acac)3. See DOI: 10.1039/c2ra21277c |
| ‡ Present address: Nanomaterials Research Unit, SAM Department, Centre de Recherche Public-Gabriel Lippmann, L-4422 Belvaux, Luxembourg. |
| This journal is © The Royal Society of Chemistry 2012 |