High capacity rechargeable battery electrode based on mesoporous stacked Mn3O4 nanosheets†
Deepak P. Dubal and Rudolf Holze*
Technische Universität Chemnitz, Institut für Chemie, AG Elektrochemie, D-09107 Chemnitz, Germany. E-mail: rudolf.holze@chemie.tu-chemnitz.de; dubaldeepak2@gmail.com
First published on 8th October 2012
Abstract
Novel stacked nanosheets of Mn3O4 thin films were synthesized on a large scale by a facile and efficient low-temperature chemical bath deposition (CBD) route, without templates or surfactants. The aligned nanosheets have a high surface area and a mesoporous structure, which were expected to help to improve the electrochemical property in Li+ batteries. This synthetic procedure is straightforward, inexpensive and thus facilitates mass production of Mn3O4 stacked nanosheets.
The automobile market is presently aimed toward the development of low emission cars, such as hybrid electric vehicles (HEVs) and plug-in hybrid electric vehicles (PHEVs), and of zero emission, full electric vehicles (EVs).1 Making these sustainable vehicles a reality still depends on the availability of suitable energy storage systems such as high energy lithium ion batteries. Great efforts have been devoted to develop different types of materials with high reversible capacity, long cycle life, and low cost.2 Electrochemically active metal oxides such as SnO2, Fe2O3, Co3O4, MnO2 and NiO have long been considered as promising anode materials for lithium ion batteries because of their higher theoretical capacities and high energy densities than those of conventional graphite anodes (372 mAh g−1).3 However, these materials suffer from poor stability due to the pulverization process, although efforts have been taken to improve the cyclability and specific capacity through nanostructuring transition metal oxides.4
Among different nanostructured transition metal oxides, manganese oxides are some of the most attractive materials for battery electrode due to their low cost, great environmental compatibility and good specific capacity.5 There are different polymorphs of manganese oxide (MnO, Mn3O4, MnO2), among them Mn3O4 undergoes relatively little research. However, it plays important roles in energy storage, catalysts, soft magnetic materials etc., and has recently drawn growing attention.6 As far as we know, only a few reports were related to the Li-ion battery properties of Mn3O4 material. Surprisingly, previous reports of Mn3O4 have suggested that the material has poor lithiation activity, despite being isostructural with Co3O4. In one report, pure Mn3O4 was shown to have a reversible capacity of just 200 mAh g−1, whereas a cobalt-doped sample of Mn3O4 exhibited a stable reversible capacity of 400 mAh g−1 with a first coulomb efficiency of 45%.7 The activity of electrode materials is primarily determined by the microstructure of active species. Various nanostructures of Mn3O4 such as nanorods/nanowires, mesoporous/hollow spheres, nanofibers have been synthesized by different routes, such as solid state reaction, chemical bath deposition and γ-ray irradiation.8 Especially, CBD method as a typical solution-based approach has been proven to be an effective and convenient process in preparing various inorganic materials with diverse controllable morphologies and architectures directly on the substrates.9
Herein, a mild, simple and scalable strategy has been developed to realize the synthesis of self-assembly of stacked layers of Mn3O4 nanosheets (2D). The size and crystalline nature of the stacked nanosheets layers can be tuned easily by changing the concentration of complexing agent. The results indicate that this type of Mn3O4 exhibits a high initial capacity of 824 mAh g−1. The Li storage performance of Mn3O4 electrodes was much better than those reported for manganese oxide based anode materials. High surface area and a mesoporous structure of well-arranged stacked nanosheets help to improve the electrochemical property in Li+ batteries. Although the specific capacity reported here is lower than the previously reported values for Mn3O4, this material can be used as new and promising electrode material in battery application due to better stability and high rate capability.
Synthesis of stacked nanosheets of Mn3O4 thin films by CBD method is based on immersing the titanium substrates into an aqueous solution of manganese sulfate complexed with hexamethylenetetramine (HMT). Firstly, solutions of 0.1 M manganese sulfate as a source of manganese with two different concentrations of HMT (0.05 and 0.1 M) were prepared. Well cleaned titanium substrates were immersed in these above prepared two baths placed at temperature of 343 K. When the bath attained the temperature of 343 K, the brownish precipitation started in the bath. During precipitation, a heterogeneous reaction occurred and Mn3O4 was deposited on the substrates. The deposition time for Mn3O4 sample placed at 343 K is kept constant at 3 h for both baths. The films were annealed at 473 K for 2 h, in order to remove hydroxide and to improve the crystallinity of deposited films. The films obtained after the deposition period of 3 h for the HMT concentration of 0.05 and 0.1 M are hereafter symbolized as M1 and M2, respectively. For experimental set up see ESI, Fig. S1.†
Structural identification of Mn3O4 films were carried out using a Rigaku Rotalflex RU-200B diffractometer using a Cu-Kα (λ = 1.5418 Å) source. The microstructures of films were examined with a field emission scanning electron microscope (FESEM JEOL JSM-7500F). TEM and HRTEM observations were conducted using JEOL JEM-2100 operated at 200 kV. Electrochemical measurements were conducted in coin cells. The Mn3O4 electrode film was directly deposited on to titanium current collector with CBD method. It was cut into circular electrodes. The coin cells were assembled by employing a composite electrode with metallic lithium foil and 1 M LiPF6 dissolved in a solution of ethylene carbonate/dimethyl carbonate as an electrolyte in a glove box filled with argon. The cell was galvanostatically cycled between 0.01 and 3.0 V vs. Li/Li+ at various current densities.
In CBD, when the ionic product of anion and cation exceeds the solubility product, precipitation occurs and ions combine on the substrate and in the solution to form nuclei. Solid phase formation from the solution involves two steps as nucleation and particle growth. Nucleation implies that the clusters of molecules formed undergo rapid decomposition and particles combine to grow up to a certain thickness of the film. Mn3O4 thin film depositions take place on titanium substrates via slow hydrolysis of manganese sulphate solution. This can be represented as follows: Here, it is thought that HMT serves as a source of a weak reducing agent. Formaldehyde and ammonia are produced by the hydrolysis of HMT at an elevated temperature10 as shown in following equations,
| C6H12N4 + 6H2O → 6HCHO + 4NH3 | (1) |
| NH3 + H2O ⇔ NH4OH | (2) |
| MnSO4 + 2NH4OH → Mn(OH)2 + (NH4)2SO4 | (3) |
| Mn2+ + nNH3 ↔ Mn(NH3)n2+ | (4) |
| 6Mn2+ + O2 + 12OH− → 2Mn3O4 + 6H2O | (5) |
The X-ray diffraction study was carried out for the determination of crystal structure along with structural changes and identification of phases of prepared Mn3O4 thin films. Fig. 1 shows XRD patterns of polycrystalline Mn3O4 stacked nanosheets prepared at different HMT concentrations (M1 and M2) on titanium substrate. The planes corresponding to (112), (202), (220), (301), (321), (400), (105), (332), (305) and (602) are in good agreement with the standard values of tetragonal hausmannite Mn3O4 structure. The calculated lattice constants, a = 5.48 Å, b = 5.48 Å and c = 9.45 Å, are in good agreement with standard lattice constants (a = 5.7621 Å, b = 5.7621 and c = 9.4696 Å, JCPDS card no. 01-75-1560). Also from Fig. 1, it is clearly seen that as the concentration of HMT increases the crystallinity of Mn3O4 structure is also increased. The above results showed that the obtained Mn3O4 films are phase-pure and well-crystalline material. Mn3O4 is well known to have the normal spinel structure in which the Mn2+ ions occupy the tetrahedral sites while the Mn3+ ions occupy the octahedral sites. Mn3O4 also has a stable tetrahedral structure in which the oxygen octahedron is tetragonally distorted due to the Jahn–Teller effect on the Mn3+ ion.11
 | ||
| Fig. 1 XRD patterns of Mn3O4 samples at two different concentrations of HMT on titanium substrate. | ||
The corresponding morphologies and detailed structures were characterized by both FESEM and TEM analysis, as shown in Fig. 2. Fig. 2(a) shows the SEM image of the products synthesized at low HMT concentration (M1). The products look like dense stacked nanosheets assembled nearly perpendicular to the substrate. Most of the nanosheets are aggregated together (ESI, Fig. S2†) Furthermore, the stacked small nanosheets create abundant space (Fig. 2(b)), which ensures an easier electrolyte ion transport and more superficial electroactive species. When the concentration of HMT was increased, uniform stacking of Mn3O4 nanosheets (M2) can be seen in Fig. 2(c). The stacked nanosheets in sample M2 possess a larger size than that of sample M1 due to the increased HMT concentration. As the HMT concentration further increases, the stacked nanosheets grow further with increased thickness and assemble nearly perpendicular to surface (sample M2, Fig. 2(d)). The SEM image suggests that sample M2 possesses a relatively better alignment of stacked nanosheets on the surface than sample M1. However, on the basis of the observations from the TEM images (Fig. 2(e, f)), the products are actually confirmed to be loose and very thin nanosheet which is in good agreement with the XRD results. The well-resolved lattice fringes give an interplanar spacing of 0.42 nm, which is in good agreement with the distance of the (220) plane. It is reasonable that the stacked nanosheets become larger with better crystallinity when the HMT concentration increases, hence resulting in more thick stacked nanostructures.
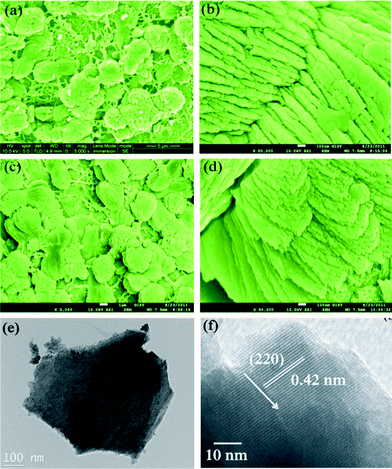 | ||
| Fig. 2 SEM images of (a–b) sample M1 and (c–d) sample M2 on titanium substrate, (e and f) TEM and HRTEM images of samples M1, respectively. | ||
A possible growth process and corresponding schematic illustration is proposed in Scheme 1. Initially, the partial hydrolysis of HMT produces formaldehyde and ammonia.12 Ammonia can form a complex with Mn2+, which decreases the free Mn2+ ion concentration and reduces the rate of crystal growth. As the reaction proceeds, more HMT hydrolyze leading to the increase of the concentration of OH− in the solution, which is favorable for the formation of thin Mn3O4 nanosheets.13 Here, HMT decomposes slowly to provide a gradual and controlled supply of ammonia. At the same time, the unreacted HMT may selectively adsorb on certain facets of Mn3O4 nanoparticles due to their high binding capacity14 resulting in the formation of nanosheets. From a thermodynamics perspective, the surface energy of an individual nanosheet is high and therefore its tendency to aggregate perpendicularly to the surface planes to decrease the surface energy is high.15 Consequently, as the reaction proceeds further, the thin sheets would self-aggregate to form Mn3O4 stacked sheet-like nanostructures for minimizing the overall surface energy. Thus as the concentration of HMT increases more thick stacked nanosheets are produced.
 | ||
| Scheme 1 Schematic depiction of the selective formation of Mn3O4 stacked nanosheets on the substrate. | ||
In order to investigate the oxidation state of Mn in Mn3O4, the XPS analyses were carried out. Fig. 3a displays the full XPS spectrum of Mn3O4 stacked nanosheets. As seen from Fig. 3a, the Mn2p3/2 peak observed at 641.48 eV and the Mn2p1/2 peak at 653.3 eV indicate that the element Mn is in +2 oxidation state (Mn2+).16 In the present case the observed spin–orbit splitting is 11.82 eV. Similar types of results are reported elsewhere.17 The O1s spectrum of Mn3O4 was analyzed by curve fitting (ESI, Fig. S3†). The spectrum is fitted with three components observed at 529.7 eV, 531.2 eV and 532.5 eV which are related to Mn–O–Mn for the tetravalent oxide, Mn–OH for hydrated trivalent oxide and H–O–H for residual water, respectively. Fig. 3b shows the nitrogen adsorption and desorption isotherms of samples and the pore size distributions of Mn3O4 sample (M1). The specific surface areas were calculated by employing the Brunauer–Emmett–Teller (BET) method and the pore size distributions (PSD) were obtained by means of the Barrett–Joyner–Halenda (BJH) equation using the adsorption isotherm branch. The adsorption behaviors of the two samples indicated typical mesoporous materials. The BET specific surface area of samples M1 and M2 were found to be 104 and 82 m2 g−1, respectively. The average pore diameter of Mn3O4 nanosheets samples are found to be in the mesoporous region, with bimodal pore size distributions. However, the pore size distribution maxima of the samples are centered at ∼3.97 and 14 nm, and 3.75 and 13.97 nm for samples M1 and M2, respectively. These results show that mesopores of different sizes originate from the spaces in interlayers and intralayers of nanosheets. Thus the mesoporous structure of stacked Mn3O4 nanosheets provides easy access for the ions in the electrolyte as well as a short diffusion path.18
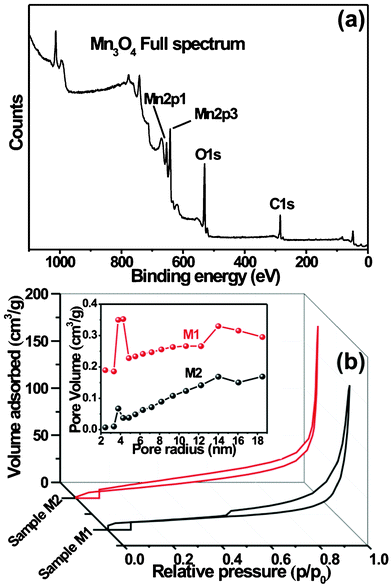 | ||
| Fig. 3 (a) Full XPS spectrum of well-ordered Mn3O4 nanosheets (sample M1). (b) Nitrogen adsorption–desorption isotherms and corresponding pore size distribution of M1 and M2 samples. | ||
Fig. 4a shows first discharge–charge profiles of both Mn3O4 samples (M1 and M2) at 0.1 C-rate in 1.0 M LiPF6 EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). The general features of the discharge–charge profiles are consistent with other transition metal oxide anodes that undergo conversion reactions, especially other manganese oxides.19 For both the samples (M1 and M2), in the first discharge curve, the potential decreases steeply to approximately 1.5 V followed by a weak slope corresponding to lithium insertion into the crystal structure of Mn3O4. When the potential decreases to about ∼0.3 V, an obvious potential plateau is observed that extends to approximately 746 mAh g−1 (sample M1) which is very near to the theoretical capacity of 937 mAh g−1 for the conversion reaction to Mn and Li2O as follows: Mn3O4 + 8Li+ + 8e− → 3Mn(0) + 4 Li2O. The sloping region at ∼1.3 V during the charge process indicates the reversible nature of this electrochemical reaction. The overall discharge capacity reaches 824 mAh g−1 and 709 mAh g−1 for samples M1 and M2, respectively, with the overall discharge capacity approximately consistent with the sloping voltage below 0.30 V. These results reveal that the morphology plays a significant role in the discharge performance, in terms of the lithium intercalation in the crystal structure of Mn3O4. When the surface area is high, the lithium ion intercalation capacity and affinity will be greatly enhanced, since the diffusion lengths of the lithium ions are greatly shortened.20 Therefore, materials with small size and high surface area always yield high discharge capacities. As has been reported for a number of transition metal oxides, the second discharge profile is different from the first one. Only one voltage plateau was observed around 0.7 V, which was distinctly higher and more sloped than the main feature of the first discharge (see Fig. 4b). This may indicate that the lithiation reaction of the second cycle is easier and is often the feature of a single phase reaction. The results showed that the Mn3O4 stacked nanosheets can serve as a potential anode material for lithium-ion batteries. This could result from the different preparation method that we developed, which gives rise to a different morphology. Although we also observed a significant irreversible capacity loss after the first cycle, such behavior is common to virtually all systems based on conversion reactions. Fig. 4c shows the discharge–charge profiles of stacked Mn3O4 nanosheets at different C-rates. It is seen that Mn3O4 shows 298 mAh g−1 capacity at high C-rate which is attributed to stacked layers of nanosheets.
1 by volume). The general features of the discharge–charge profiles are consistent with other transition metal oxide anodes that undergo conversion reactions, especially other manganese oxides.19 For both the samples (M1 and M2), in the first discharge curve, the potential decreases steeply to approximately 1.5 V followed by a weak slope corresponding to lithium insertion into the crystal structure of Mn3O4. When the potential decreases to about ∼0.3 V, an obvious potential plateau is observed that extends to approximately 746 mAh g−1 (sample M1) which is very near to the theoretical capacity of 937 mAh g−1 for the conversion reaction to Mn and Li2O as follows: Mn3O4 + 8Li+ + 8e− → 3Mn(0) + 4 Li2O. The sloping region at ∼1.3 V during the charge process indicates the reversible nature of this electrochemical reaction. The overall discharge capacity reaches 824 mAh g−1 and 709 mAh g−1 for samples M1 and M2, respectively, with the overall discharge capacity approximately consistent with the sloping voltage below 0.30 V. These results reveal that the morphology plays a significant role in the discharge performance, in terms of the lithium intercalation in the crystal structure of Mn3O4. When the surface area is high, the lithium ion intercalation capacity and affinity will be greatly enhanced, since the diffusion lengths of the lithium ions are greatly shortened.20 Therefore, materials with small size and high surface area always yield high discharge capacities. As has been reported for a number of transition metal oxides, the second discharge profile is different from the first one. Only one voltage plateau was observed around 0.7 V, which was distinctly higher and more sloped than the main feature of the first discharge (see Fig. 4b). This may indicate that the lithiation reaction of the second cycle is easier and is often the feature of a single phase reaction. The results showed that the Mn3O4 stacked nanosheets can serve as a potential anode material for lithium-ion batteries. This could result from the different preparation method that we developed, which gives rise to a different morphology. Although we also observed a significant irreversible capacity loss after the first cycle, such behavior is common to virtually all systems based on conversion reactions. Fig. 4c shows the discharge–charge profiles of stacked Mn3O4 nanosheets at different C-rates. It is seen that Mn3O4 shows 298 mAh g−1 capacity at high C-rate which is attributed to stacked layers of nanosheets.
 | ||
Fig. 4 (a) Discharge–charge profiles of two Mn3O4 samples. (b) First and second discharge–charge profiles of sample M1 at current rate of 0.1 C (c) Discharge–charge profiles of sample M1 at different C-rates. (c) Cycle performance of Mn3O4 at a current rate of 0.1 C in 1.0 M LiPF6 EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). 1 by volume). | ||
Fig. 4d presents the changes in discharge–charge capacity of Mn3O4 with cycling at a rate of 0.1 C. It can be seen that the cycling is quite stable. The reversible capacity exhibits almost no significant fading after 60 cycles. Better electrochemical performance of the synthesized Mn3O4 could be attributed to its special stacked architecture. The nanoscale provides large surface area, which improves the utilization of active material, and the loose porous nanosheets-like structure allows lithium ions to transfer easily in and out. The material is also able to accommodate the strain induced by possible volume change during discharge–charge process and maintain the integrity of the electrode. This may be responsible for the good cycling stability.
Conclusions
In conclusion, simple and scalable CBD method has been developed to synthesize well-arranged stacked Mn3O4 nanosheets with different sizes directly on to titanium substrate. Based on these stacked nanostructures, the relationships between the specific area and the electrochemical performances have been discussed in our work. Among two samples, the sample synthesized at low concentration of HMT, with an especially high specific area (104 m2 g−1), uniform pore size distribution and poor crystallinity, demonstrated the good specific capacity as well as cycling stability. Electrochemical measurements indicate that the well-ordered stacked Mn3O4 nanosheets shows a high initial capacity 824 mAh g−1 and a stabilized reversible capacity of around 400 mAh g−1 upon cycling. The electrochemical reaction becomes highly reversible after the first discharge, and the reversible capacity exhibits almost no significant fading after 60 cycles. The excellent electrochemical performance of this specific Mn3O4 could be attributed to its special stacked architecture.Acknowledgements
One of the authors (D.P.D.) appreciates the award of a Humboldt Fellowship of the Alexander von Humboldt Foundation (AvH), Germany.References
- (a) Advances in Lithium-ion Batteries, ed. W. A. van Schalkwijk and B. Scrosati, Kluwer Academic/Plenum Publishers, New York, 2002 Search PubMed; (b) J. Hassoun, K. S. Lee, Y. K. Sun and B. Scrosati, J. Am. Chem. Soc., 2011, 133, 3139 CrossRef CAS.
- (a) P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS; (b) C. Wang, Y. Zhou, M. Y. Ge, X. B. Xu, Z. L. Zhang and J. Z. Jiang, J. Am. Chem. Soc., 2010, 132, 46 CrossRef CAS.
- (a) C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Chem. Mater., 2005, 17, 3297 CrossRef CAS; (b) N. Li, G. Liu, C. Zhen, F. Li, L. Zhang and H.-M. Cheng, Adv. Funct. Mater., 2011, 21, 1717 CrossRef CAS.
- C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Chem. Mater., 2005, 17, 3297 CrossRef CAS.
- (a) B. Li, G. Rong, Y. Xie, L. Huang and C. Feng, Inorg. Chem., 2006, 45, 6404 CrossRef CAS; (b) C. D. Lokhande, D. P. Dubal and O. S. Zoo, Curr. Appl. Phys., 2011, 11, 255 CrossRef.
- (a) L. Ji, A. Medford and X. Zhang, J. Mater. Chem., 2009, 19, 5593 RSC; (b) Y. G. Wang, L. Chen, F. Li, H. M. Xiong and Y. Y. Xia, Chem. Mater., 2007, 19, 2095 CrossRef CAS.
- D. Pasero, N. Reeves and A. R. West, J. Power Sources, 2005, 141, 156 CrossRef CAS.
- (a) P. Li, C. Y. Nan, Z. Wei, J. Lu, Q. Peng and Y. D. Li, Chem. Mater., 2010, 22, 4232 CrossRef CAS; (b) M. Fang, X. L. Tan, M. Liu, S. H. Kang, X. Y. Hu and L. D. Zhang, CrystEngComm, 2011, 13, 4915 RSC.
- (a) V. R. Shinde, H. S. Shim, T. P. Gujar, H. J. Kim and W. B. Kim, Adv. Mater., 2008, 20, 1008 CrossRef CAS; (b) D. P. Dubal, V. J. Fulari and C. D. Lokhande, Microporous Mesoporous Mater., 2012, 51, 511 CrossRef.
- X. Gao, X. Li and W. Yu, J. Phys. Chem. B, 2005, 109, 1155 CrossRef CAS.
- (a) H. Y. Xu, S. L. Xu, X. D. Li, H. Wang and H. Yan, Appl. Surf. Sci., 2006, 252, 4091 CrossRef CAS; (b) A. S. Fritsch, J. Sarrias, A. Rousset and G. U. Kulkarni, Mater. Res. Bull., 1998, 33, 1185 CrossRef.
- (a) L. E. Greene, B. D. Yuhas, M. Law, D. Zitoun and P. D. Yang, Inorg. Chem., 2006, 45, 7535 CrossRef CAS; (b) H. Wang, J. Q. Xu and Q. Y. Pan, CrystEngComm, 2010, 12, 1280 RSC.
- C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772 RSC.
- (a) F. Chen, R. J. Zhou, L. G. Yang, N. Liu, M. Wang and H. Z. Chen, J. Phys. Chem. C, 2008, 112, 1001 CrossRef CAS; (b) X. D. Gao, X. M. Li and W. D. Yu, J. Phys. Chem. B, 2005, 109, 1155 CrossRef CAS.
- L. X. Yang, Y. J. Zhu, H. Tong, Z. H. Liang and W. W. Wang, Cryst. Growth Des., 2007, 7, 2716 CAS.
- P. S. Shah, J. D. Holmes, K. P. Johnston and B. A. Korgel, J. Phys. Chem., 2002, 106, 2545 CrossRef CAS.
- R. J. Iwanowski, M. H. Heinonen and E. Janik, Chem. Phys. Lett., 2004, 387, 110 CrossRef CAS.
- M. W. Xu, L. B. Kong, W. J. Zhou and H. L. Li, J. Phys. Chem. C, 2007, 111, 19141 CAS.
- (a) Q. Fan and M. S. Whittingham, Electrochem. Solid-State Lett., 2007, 10, A48 CrossRef CAS; (b) J. Zhao, Z. Tao, J. Liang and J. Chen, Cryst. Growth Des., 2008, 8, 2799 CrossRef CAS; (c) K. Zhong, X. Xia, B. Zhang, H. Li, Z. Wang and L. Chen, J. Power Sources, 2010, 195, 3300 CrossRef CAS.
- H. Wang, L.-F. Cui, Y. Yang, H. Sanchez Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental set up, SEM, XPS spectrum. See DOI: 10.1039/c2ra21806b |
| This journal is © The Royal Society of Chemistry 2012 |
