Electron impact on N2/CH4 mixtures in He droplets—probing chemistry in Titan's atmosphere†
Sylwia
Ptasinska
*ab,
Iogann
Tolbatov
ab,
Peter
Bartl
c,
James
Yurkovich
ad,
Benjamin
Coffey
ad,
Daniel M.
Chipman
a,
Christian
Leidlmair
c,
Harald
Schöbel
c,
Paul
Scheier
c and
Nigel J.
Mason
e
aRadiation Laboratory, University of Notre Dame, Notre Dame, IN, USA. E-mail: sptasins@nd.edu; Fax: (+1)-574-631-8068
bDepartment of Physics, University of Notre Dame, Notre Dame, IN, USA
cInstitut für Ionenphysik and Angewandte Physik and Center of Molecular Bioscience Innsbruck, Universität Innsbruck, Technikerstraße 25, A-6020 Innsbruck, Austria
dDepartment of Electrical Engineering, University of Notre Dame, Notre Dame, IN, USA.
eDepartment of Physics and Astronomy, The Open University, Milton Keynes, MK7 6AA, United Kingdom
First published on 26th September 2012
Abstract
In this work a low-energy electron beam is used to irradiate mixtures of nitrogen and methane in helium droplets. The formation of heterogeneous ionic species due to ionization is observed and the corresponding chemical structures and binding energies are calculated. Formation of a CN bond in CH3N2+ is shown computationally, which indicates the possibility of synthesis of true chemical bonds. The formation of CN bonds in organic molecules is of relevance for understanding atmospheric chemistry, for example in Titan's atmosphere.
The atmosphere of Titan is mainly composed of nitrogen (N2) with a small fraction of the atmosphere consisting of other gases, e.g., methane (CH4). Indeed methane was the first hydrocarbon identified in Titan's atmosphere by telescopic observation of its infra-red (IR) spectra.1 Later, ground based and spacecraft observation of the stratosphere of Titan revealed more organic constituents, particularly heavier hydrocarbons and nitriles.2–7 Such a diverse mixture of compounds is indicative of complex chemistry taking place in Titan's atmosphere. The Cassini/Huygens mission to Saturn in 2004 provided the most detailed results on the nature of Titan's environment by direct atmospheric measurements of the abundance of molecular constituents at different altitudes.8 The gas chromatograph–mass spectrometer (GCMS) on the Huygens probe detected 1.4% CH4 at higher altitude and less than 0.5% molecular hydrogen (H2).8 The amount of CH4 increases to 5% toward Titan's surface. Both, the computational simulation9,10 and the measurement11 of the electron and ion conductivities revealed the existence of an ion-rich layer with a high amount of free electrons produced from cosmic rays.
Complex chemistry within a lunar atmosphere is unique to Titan in the solar system and has stimulated many experimental and theoretical groups to simulate and model the organic chemistry on Titan in order to understand the origin of all the compounds that have been observed.12 Recent studies of low-temperature branching ratios for the reaction of state-prepared N2+ with acetonitrile13 and with methane, acetylene and ethylene14 provide experimental evidence for the kind of ion–molecule reactions that are necessary to understand Titan's nitrile chemistry. The theoretical basis for reactions between the main constituents of Titan's atmosphere—methane,15–17 molecular hydrogen and nitrogen,15 and the Prussic acid cations16—is also being developed and used in models of Titan's atmosphere.
Nevertheless, many reaction pathways have yet to be revealed. To date, most physical and chemical processes have been attributed to interactions between photons and atmospheric compounds.18,19 However, the presence of electrons produced from cosmic rays would also influence the chemistry occurring in Titan's atmosphere.
In this study, we attempt to understand the formation of ionic species by a novel experimental approach, where a mixture of Titan's main constituents (N2 and CH4) is embedded in He nanodroplets and irradiated with a low-energy electron beam (70 eV). Such low-energy electrons can be produced in Titan's atmosphere as a consequence of photo-ionization of the atmospheric molecules. Helium droplets provide an ultra-cold environment (0.37 K) which is much colder than Titan's atmosphere; however, this is an ideal inert medium to study the ability of ionic species to trigger chemical reactions leading to the formation of new products whilst simultaneously neglecting any temperature effects.
The experimental measurements were carried out using a commercial time-of-flight mass spectrometer (Tofwerk, H-TOF) coupled with a Nier-type ion source and a home built helium (He) droplet source.20 More details of the experimental methods are contained in the Electronic Supplementary Information.†
Mass spectra up to 250 daltons (Da) were obtained from both pure gases and from a gas mixture with a N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 ratio of 9
CH4 ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 embedded in He droplets (see Fig. 1).
1 embedded in He droplets (see Fig. 1).
 | ||
| Fig. 1 A direct comparison of three mass spectra for cations produced from 70 eV electron impact on He droplets doped with N2 (black curve), CH4 (red curve) or a N2/CH4 mixture (green curve). Asterisks indicate peaks detected only in the case of N2/CH4 mixtures. | ||
Ionization of the dopants can be performed mainly via formation of He+ and subsequent resonant charge transfer.21 In the case of pure gases, N2 or CH4, the most intense series of peaks are assigned to homogeneous molecular clusters (N2)n+ and (CH4)m+/(CH4)mH+ with a number of units up to n = 8 and m = 15, respectively. The mass spectrum from the mixture of both gases exhibits strong cluster formation of heterogeneous species, indicated in Fig. 1 by asterisks.
In contrast, the yields of both (N2)n+ and (CH4)m+ are lower in the case of mixed gases than in those obtained in pure gases. In this Communication we will focus on heterogeneous species, which are formed from both molecules, with the highest observed ion yields.
Fig. 2 represents three portions of the high resolution mass spectra centered on the heterogeneous dimer region at the nominal masses of 43, 44 and 45 Da, showing the highest signal for ionic complexes which are stable in the time window of the present measurement. He cluster ions and some other ions formed due to impurities in the system, such as CO2, were observed in addition to strong peaks corresponding to CH3N2+, CH4N2+ and CH5N2+. The GCMS on the Huygens probe was in direct contact with Titan's surface and also recorded cations in this mass range.8 Therefore, we will concentrate in this work mainly on these three species.
 | ||
| Fig. 2 High resolution spectra for cations produced from 70 eV electron impact to He droplets doped with N2 (black curve), CH4 (red curve) or a N2/CH4 mixture (green curve) at nominal masses of 43 Da (upper graph), 44 Da (middle) and 45 Da (bottom). | ||
Additionally, we have performed computational modelling of all cationic species that might reasonably be expected to be detected over the mass range for dimers. Calculations were made at the CCSD(T)/cc-pVTZ//CCSD/6-311G(2d,p) level, and include corrections for zero point vibrational energy differences and for basis set superposition errors. More details of the methods are contained in the Electronic Supplementary Information.† Many different ways of having the two interacting species approach one another were considered, leading to only a small number of local minimum structures which are shown in Fig. 3.
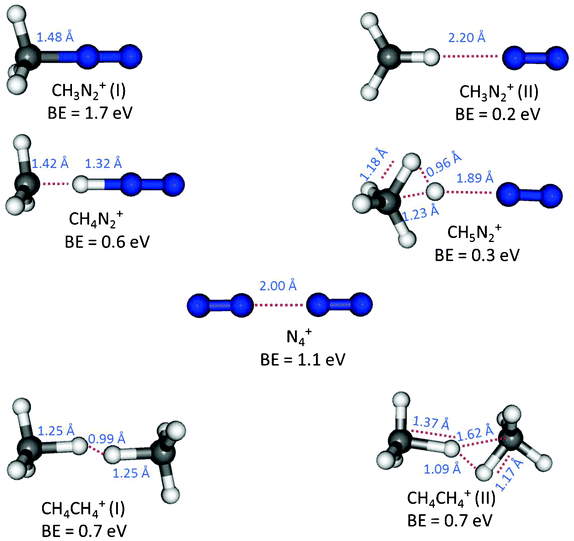 | ||
| Fig. 3 Local minimum structures and binding energies, including corrections for zero point vibrational energy differences and for basis set superposition errors, as calculated with the CCSD(T)/cc-pVTZ//CCSD/6-311G(2d,p) method. Particularly notable interatomic distances are also shown. | ||
The homogeneous clusters were each found to have substantial binding energy (BE), with the CH4CH4+ species having two local minimum structures (distinguished by appending a Roman numeral in parentheses). These structures were found to each have a BE of 0.7 eV, while the N4+ species has a BE of 1.1 eV. In all of the homogeneous clusters, the positive charge is equally divided between the two interacting units. The BE found for N4+ is in good agreement with experimental results22 and the structure found agrees well with previous high-level calculations.23,24 For heterogeneous clusters, computational results are reported for N2 interacting with CH3+, CH4+, and CH5+. In each case, the charge in the complex remains predominantly on the CHm+ fragment, albeit with some delocalization onto the N2 fragment. The other possible asymptote of N2+ interacting with CH3, CH4, or CH5 was found to be considerably less stable.
In our experiment, the peak area for the CH3N2+ ion, which is formed by hydrogen loss from the ionized methane unit in heterogeneous clusters, is the largest among the reported ions. Two local minimum structures were found for CH3N2+. The most stable of these structures, CH3N2+(I), is particularly notable; its large BE (1.7 eV) and short CN separation (1.48 Å) is similar to CN bonds in typical organic molecules and indicates the synthesis of a true chemical bond between the units. This computed binding energy is in good agreement with an experimental determination25 and the structure found agrees well with a previous high-level calculation.26 This very stable structure is undoubtedly mainly responsible for the large peak area observed for the CH3N2+ ion. In contrast, the CH3N2+(II) structure has low BE and large separation between the units, indicating this structure should, at best, be regarded as a weakly interacting complex. The second most intense peak in the mass spectrum found at 44 Da is assigned to the CH4N2+ ion, where only one local minimum structure was found, with a shared hydrogen actually being slightly closer to nitrogen than to carbon. It has relatively low BE and large separation between the units, indicating that it should be regarded as a weakly interacting complex. The same comment also applies to the single structure found for the CH5N2+ ion, a product which is based upon a protonated methane (CH5+) that is formed via proton transfer from a charged methane unit within a (CH4)m+ cluster.
A pronounced peak was also detected at a mass of 44 Da by the GCMS on the Huygens probe8 and was present in all spectra taken at the surface and different altitudes of Titan. This mass was previously attributed to the CO2 molecule, while in our experiments the most abundant ion formed in this mass range is CH4N2+ and only a low signal was observed for CO2. In our experiment, the assignment of CH4N2+, rather than CO2, as the main contributor to the peak at 44 Da is primarily due to the high mass resolution of the mass spectrometer, but it does not exclude the presence of CO2 in Titan’s atmosphere.
Further analysis of different compounds showed that the highest production of these larger ionic structures corresponds with a particular N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 atmospheric ratio. Fig. 4 shows three different ion yields as a function of the N2
CH4 atmospheric ratio. Fig. 4 shows three different ion yields as a function of the N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 ratio. The yield of CH4N2+ ions was highest at a 95
CH4 ratio. The yield of CH4N2+ ions was highest at a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio and decreases with a higher concentration of methane in the mixture. Similar trends were observed for two other compounds, CH3N2+ and CH5N2+, with maxima in the range of 10–20% and 30–40% of CH4 in the mixture, respectively. This indicates the complexity of chemical reactions in such mixtures. We will, therefore, continue this novel experimental approach to study larger heterogeneous complexes using different ratios of both gases in order to deduce possible reactions responsible for the formation of bonds in such mixtures.
5 ratio and decreases with a higher concentration of methane in the mixture. Similar trends were observed for two other compounds, CH3N2+ and CH5N2+, with maxima in the range of 10–20% and 30–40% of CH4 in the mixture, respectively. This indicates the complexity of chemical reactions in such mixtures. We will, therefore, continue this novel experimental approach to study larger heterogeneous complexes using different ratios of both gases in order to deduce possible reactions responsible for the formation of bonds in such mixtures.
 | ||
Fig. 4 Ion yields as function of N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 ratio for three different heterogeneous compounds. CH4 ratio for three different heterogeneous compounds. | ||
Conclusions
It has very recently been reported that Titan fulfils all required conditions for the origin of life, i.e., it is thermodynamically unstable, it comprises rich carbon-containing molecules, and there is evidence of liquid substances at the surface27 and also the existence of a water ocean under a thick layer of ice.28 Therefore, the knowledge of heterogeneous structure formation, particularly molecules containing CN bonds,29 is important in understanding the synthesis and nature of organic species which can be formed in Titan's atmosphere. Formation of DNA nucleobases on Titan aerosol analogs exposed to X-ray irradiation has been observed in another laboratory based experiment.30 Several organic molecules, including nitriles and aromatic CN compounds, were detected as products of X-rays and secondary electrons in molecular ices consisting of N2 and CH4 with some traces of H2O and CO2. It was suggested that such production of prebiotic compounds can be used as an analogue of early Earth.30 Therefore, we were intrigued by the possibility of organic molecule formation in Titan's atmosphere, particularly due to interactions between ionic species formed from N2 and CH4 gas mixtures. Our unusual approach of studying gas-phase chemistry at very low temperature regimes, that is in He droplets, has shown the production of many heterogeneous clusters. In order to understand their nature, we have performed ab initio calculations of binding energies of complexes observed at high abundances in our experiment. These calculations have shown that for some structures, e.g., CH3N2+, the chemical bond can be formed. We are aware of the fact that our experiment and calculations neglected the elevated temperatures which are present in Titan's atmosphere. Therefore, in further experimentation we would like to include temperature effects on the process of bond formation in N2/CH4 mixtures.Acknowledgements
The research described herein was supported by the Division of Chemical Sciences, Geosciences and Biosciences, Basic Energy Sciences, Office of Science, United States Department of Energy (DOE) through Grant No. DE-FC02-04ER15533, and the FWF (P19073). This is contribution number NDRL 4911 from the Notre Dame Radiation Laboratory. P.B. gratefully acknowledges a dissertation grant from the vice-rector for research of the University of Innsbruck. S.P. would like to thank the COST action (CM0601-6261) for her scientific visit in Innsbruck.References
- G. P. Kuiper, Astrophys. J., 1944, 100, 378 CrossRef.
- F. C. Gillett, W. J. Forrest and K. M. Merrill, Astrophys. J., 1973, 184, L93 CrossRef CAS.
- F. C. Gillett, Astrophys. J., 1975, 201, L41 CrossRef CAS.
- A. Tokunaga, S. Beck, T. Geballe and J. Lacy, Bulletin of the American Astronomical Society, 1980, 12, 669 Search PubMed.
- W. C. Maguire, R. A. Hanel, D. E. Jennings, V. G. Kunde and R. E. Samuelson, Nature, 1981, 292, 683 CrossRef CAS.
- V. G. Kunde, A. C. Aikin, R. A. Hanel, D. E. Jennings, W. C. Maguire and R. E. Samuelson, Nature, 1981, 292, 686 CrossRef CAS.
- R. Hannel, B. Conrath, F. M. Flasar, V. Kunde, W. Maguire, J. Pearl, J. Pirraglia, R. Samuleson, L. Herath, M. Allison, D. Cruikshank, D. Gautier, P. Gierasch, L. Horn, R. Koppany and C. Ponnamperuma, Science, 1981, 212, 192 Search PubMed.
- H. B. Niemann, S. K. Atreya, S. J. Bauer, G. R. Carignan, J. E. Demick, R. L. Frost, D. Gautier, J. A. Haberman, D. N. Harpold, D. M. Hunten, G. Israel, J. I. Lunine, W. T. Kasprzak, T. C. Owen, M. Paulkovich, F. Raulin, E. Raaen and S. H. Way, Nature, 2005, 438, 779 CrossRef CAS.
- W. J. Borucki, Z. Levin, R. C. Whitten, R. G. Keesee, L. A. Capone, A. L. Summers, O. B. Toon and J. Dubach, Icarus, 1987, 72, 604 CrossRef CAS.
- W. J. Borucki, R. C. Whitten, E. L. O. Bakes, E. Barthc and S. Tripathi, Icarus, 2006, 181, 527 CrossRef CAS.
- G. J. Molina-Cuberos, J. J. Lopez-Moreno, R. Rodrigo, K. Schwingenschuh, N. Thomas, A. Coustenis, J. Leliwa, J. Kopysty and K.J. Kossacki, Adv. Space Res., 2001, 28, 1511 CrossRef.
- F. Raulin, C. Brassé, O. Poch and P. Coll, Chem. Soc. Rev., 2012, 41, 5380 RSC.
- W. K. Gichuhi and A. G. Suits, J. Phys. Chem. A, 2012, 116, 938 CrossRef CAS.
- W. K. Gichuhi and A. G. Suits, J. Phys. Chem. A, 2011, 115, 7105 CrossRef CAS.
- I. Tanarro, V. J. Herrero, A. M. Islyaikin, I. Mendez, F. L. Tabares and D. Tafalla, J. Phys. Chem. A, 2007, 111, 9903 CrossRef.
- Y. Li, H. Liu, X. Huang, C. Geng, C. Sun and A. Tang, J. Phys. Chem. A, 2008, 112, 2693 CrossRef CAS.
- N. Balucani, A. Bergeat, L. Cartechini, G. G. Volpi, P. Casavecchia, D. Skouteris and M. Rosi, J. Phys. Chem. A, 2009, 113, 11138 CrossRef CAS.
- M. Dobrijevic, E. Hébrard, S. Plessis, N. Carrasco, P. Pernot and M. Bruno-Claeys, Adv. Space Res., 2010, 45, 77 CrossRef.
- Z. Peng, M. Dobrijevic, E. Hébrard, N. Carrasco and P. Pernot, Faraday Discuss., 2010, 147, 137 RSC.
- H. Schöbel, C. Leidlmair, P. Bartl, A. Aleem, M. Hager, O. Echt, T. D. Märk and P. Scheier, Phys. Chem. Chem. Phys., 2011, 13, 1092 RSC.
- A. M. Ellis and S. Yang, Phys. Rev. A: At., Mol., Opt. Phys., 2007, 76, 032714–1 CrossRef.
- K. Hiraoka and G. Nakajima, J. Chem. Phys., 1988, 88, 7798 CrossRef.
- I. Carmichael, J. Phys. Chem., 1994, 98, 5044 CrossRef CAS.
- C. Leonard, P. Rosmus, S. Carter and N. C. Handy, J. Phys. Chem. A, 1999, 103, 1846 CrossRef CAS.
- M. N. Glukhovtsev, J. E. Szulejko, T. B. McMahon, J. W. Gauld, A. P. Scott, B. J. Smith, A. Pross and L. Radom, J. Phys. Chem., 1994, 98, 13099 CrossRef CAS.
- D. Dixon, W. A. de Jong, K. A. Peterson and T. B. McMahon, J. Phys. Chem. A, 2005, 109, 4073 CrossRef CAS.
- L. H. Norman and A. D. Fortes, Astronomy and Geophysics, 2011, 52, 39 CAS.
- R. A. Kerr, Science, 2012, 336, 1629 CrossRef CAS.
- N. Balucani, Chem. Soc. Rev., 2012, 41, 5473 RSC.
- S. Pilling, D. P. P. Andrade, Á. C. Neto, R. Rittner and A. Naves de Brito, J. Phys. Chem. A, 2009, 113, 11161 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21444j |
| This journal is © The Royal Society of Chemistry 2012 |
