Tunable/switchable one-dimensional photonic crystals based on a multilayer architecture of layered double hydroxides and titanium dioxide†
Jingbin
Han
,
Yibo
Dou
,
Min
Wei
*,
David G.
Evans
and
Xue
Duan
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: weimin@mail.buct.edu.cn.; Fax: 86-10-64425385; Tel: 86-10-64412131
First published on 7th September 2012
Abstract
LDH/TiO2 one-dimensional photonic crystals (1DPCs) covering the whole visible light range were fabricated by alternate deposition technique. The switchable color of LDH/TiO2 1DPCs can be achieved by repeating calcination–rehydration cycles, as a result of the associated changes in thickness and refractive index of the LDH slabs.
One-dimensional photonic crystals (1DPCs), also known as Bragg reflectors have a diverse range of applications, including ultra-high reflectivity mirrors and frequency selective filters. Their optical properties arise from the periodic modulation in refractive index n of alternating layers with high (nH) and low (nL) refractive indices.1–3 Films composed of such stacks exhibit brilliant colors when their one-dimensional photonic band gap (stop band) falls in the visible region, responsible for their application as effect pigments.4,5 For applications in displays, sensors and information storage, however, it is necessary to develop next-generation smart 1DPCs in which the position of the stop band can be tuned or switched as required. The way in which this might be achieved follows from the equation6
| λmax = 2(nLdL + nHdH) | (1) |
Conventional 1DPCs are fabricated by spin-coating or layer-by-layer assembly of pairs of oxides which possess a very large difference in refractive index, such as SiO2 (n = 1.45) and TiO2 (n = 2.44).7–9 While the stop band of such materials can be tuned by changing the layer thickness of the individual components (dL or dH), this obviously does not provide a means of reversible modulation of the wavelength. Although the refractive indices of materials such as pure SiO2 and TiO2 are fixed, there have been a few reports of reversibly tunable 1DPCs based on the reversible modulation of the effective refractive indices of nanoporous SiO2 and TiO2 by repeated infiltration and extraction of solvents or dyes from the nanopores.10,11 A more robust and environmentally-friendly device should be obtainable by replacing either SiO2 or TiO2 with a single material which undergoes a reversible change in refractive index as a result of a reversible structural transformation.
Layered double hydroxides (LDHs) or hydrotalcites, are layered anionic clays generally expressed by the formula [M2+1−xM3+x(OH)2](An−)x/n·mH2O, where M2+ and M3+ are metal cations and An− is a charge-compensating anion.12–14 Recently, LDH nanoparticles have been used as building blocks to fabricate nanoscale composite materials, which show interesting photoluminescent and mechanical properties.15 Unlike other types of clays (such as montmorillonite, kaolinite and illite), LDHs can undergo a reversible structural change, since calcination at moderate temperatures leads to the formation of mixed metal oxides (MMOs), which on subsequent rehydration regenerate the LDH phase.16 Here we show how this unique feature of LDHs can be exploited to fabricate a new 1DPC based on a multilayer system composed of LDH and TiO2 nanoparticles. The optical response of the 1DPC can be tailored either by changing the physical thickness of the individual slabs or by tuning the refractive index through triggering the structural transformation between LDHs and MMOs.
Stable colloidal suspensions of anatase TiO2 (particle size ∼10 nm) and MgAl–NO3–LDH with a basal spacing of ∼8.7 Å (particle size ∼40 nm) were synthesized according to the literature and characterized by transmission electron microscopy (TEM) and X-ray diffraction (XRD) (Fig. S1, ESI†). The 1DPCs were fabricated on Si wafers by alternate deposition of LDH and TiO2 nanoparticles via the spin-coating technique, with a TiO2 layer as outermost slab. The thickness of both slabs can be tailored by appropriate choice of coating parameters (Fig. S2, ESI†). A field emission scanning electron microscope (SEM) image (Fig. 1A) of the LDH slab shows that the LDH platelets tend to stack face-to-face. A cross-sectional image of a six-slab LDH/TiO2 multilayer film shows well-defined interfaces between the two components (inset of Fig. 1A). The average thicknesses of the TiO2 and LDH slabs are 142 ± 16 and 156 ± 20 nm, respectively. Atomic force microscopy (AFM) measurements (Fig. S3, ESI†) give a root-mean-square (RMS) roughness of 15–23 nm, indicating that the LDH/TiO2 multilayer films have a relatively smooth surface. Fig. 1B shows that the reflectance intensity of the LDH/TiO2 stacks increases monotonically with slab number, as expected from the following equation:17
 | (2) |
 | ||
| Fig. 1 (A) Field emission SEM image of LDH slab as the first layer; the inset shows a cross-sectional SEM image of six-slab LDH/TiO2 multilayer film; (B) evolution of the reflectance spectra of the LDH/TiO2 films with the number of stacked slabs (N). | ||
Tailoring of the optical response of the LDH/TiO2 1DPC was achieved by varying the thickness of the LDH slabs, while keeping the TiO2 slabs at a constant thickness (Fig. 2A). In all cases, the well-defined shape and high intensity of the reflectance band (Fig. 2B) demonstrates the excellent optical quality of the multilayer stacks. The maximal stop bands correspond to the first-order Bragg diffraction, resulting from the constructive interference of incident beams reflected at each interface; the band position is determined by the optical thickness of the stacks. With increasing thickness of the LDH slabs, the peak position of the six-slab films shifted from 462 to 695 nm, without much variation in intensity, with a corresponding change in film color from blue to red (Fig. 2C). Likewise, the slab thickness of TiO2 could also be tailored over a wide range, allowed the position of the stop band to be tuned across the whole visible range (Fig. S4).
 | ||
| Fig. 2 (A) Schematic representation of the optical tuning of 1DPCs by means of increasing LDH slab thickness (from a to d); (B) optical spectra and (C) digital photographs showing that colors covering the full visible range could be obtained by modulating the thickness of the LDH stack in 1DPCs with N = 6 (sample size: ∼1 × 1 cm). | ||
The optical response of the LDH/TiO2 1DPC can be switched reversibly by taking advantage of the reversible phase transformation between LDH and MMO during a calcination–rehydration cycle, as schematically illustrated in Fig. 3A. The reflectance peak of the as-prepared LDH/TiO2 film (N = 6) shifted from 520 nm (Fig. 3B, curve a) to 455 nm (curve b) after the calcination process, with an accompanying visual color change from green to blue (insets a and b in Fig. 3B). When the calcined film was rehydrated through hydrothermal treatment it recovered its original color completely, with a corresponding change in its reflectance spectrum. Moreover, the reversible switching between the green and blue states was reproducible by repeated calcination–rehydration over a number of cycles (the inset c of Fig. 3B).
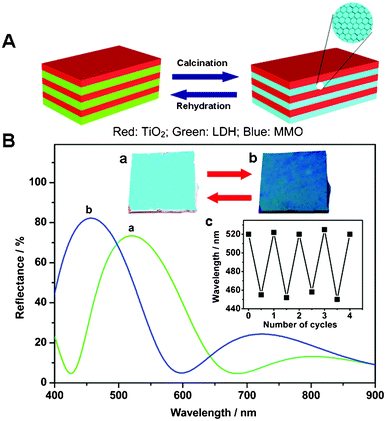 | ||
| Fig. 3 (A) Schematic illustration of the optical switching of LDH/TiO2 stacks during a calcination–rehydration cycle; (B) optical response and digital photographs showing the reversible changes in reflectance spectra and visual color. The inset c displays the reversible conversion of the stop band position with repeated calcination–rehydration cycles. | ||
In order to provide further insight into the structural changes in the LDH/TiO2 1DPC over a whole calcination–rehydration cycle, separate spin-coated LDH and TiO2 films were characterized using a spectroscopic ellipsometer (Table 1). For the LDH film, both an obvious decrease in thickness (from ∼104 to ∼94 nm) and a significant enhancement of porosity (from 14% to 39%) were observed after calcination, resulting in a marked reduction in the refractive index (from 1.53 to 1.22). After the rehydration treatment, the above three parameters all reverted to their original values. For the TiO2 film however, no obvious changes in the thickness, refractive index or porosity were found over a whole cycle indicating that, as expected, the TiO2 nanoparticles do not undergo any chemical or physical transformation. The reversible changes in the film thickness and refractive index of the LDH slabs translate into tunability of the optical thickness nLdL, which triggers the switching of the photonic stop band position, as shown by eqn (1).
| Samples | Thickness (nm) | Refractive index at 633 nm | Porosity (%)a | |
|---|---|---|---|---|
| a The ellipsometry method was utilized to determine the porosity of the LDH and TiO2 films (see the ESI† for details). | ||||
| LDH | as-prepared | 104 ± 10 | 1.53 | 14 |
| after calcination | 94 ± 10 | 1.22 | 39 | |
| after rehydration | 106 ± 10 | 1.54 | 12 | |
| TiO2 | as-prepared | 122 ± 10 | 1.96 | 11 |
| after calcination | 117 ± 10 | 1.98 | 13 | |
| after rehydration | 119 ± 10 | 1.99 | 13 | |
The phase transformations of LDH/TiO2 1DPC during a calcination–rehydration cycle were investigated by XRD (Fig. 4). The as-prepared LDH/TiO2 film (N = 6) displayed two series of reflections which can be indexed to LDH and TiO2 phases (Fig. 4a). The characteristic 003 reflection (2θ = 10.1°) of the NO3-intercalated LDH indicates a basal spacing of ∼8.7 Å, consistent with that of the as-prepared LDH suspension. Calcination at 450 °C resulted in the disappearance of the LDH phase and the appearance of peaks which can be attributed to the (200) and (220) reflections of a periclase MgO phase (Fig. 4b);18 while the TiO2 reflections remained unchanged. After rehydration, the MMO phase was reconverted to an LDH structure (Fig. 4c). The LDH (003) reflection (2θ = 12.3°) corresponding to a basal spacing of 7.2 Å indicates that an OH-intercalated LDH was formed. Both the XRD and ellipsometry results demonstrate that the calcination–rehydration cycle leads to the interconversion of LDH and MMO films accompanied by reversible changes in film thickness and refractive index, which endows the LDH/TiO2 1DPC with switchable structural color.
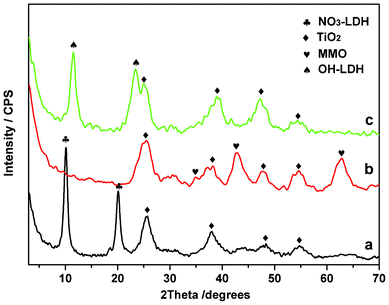 | ||
| Fig. 4 XRD patterns of (a) the as-prepared LDH/TiO2 film; (b) the MMO/TiO2 film obtained by calcination of (a); (c) the rehydrated LDH/TiO2 film obtained by hydrothermal treatment of (b). The stacking number N = 6 for all samples. | ||
Long-term stability is extremely important for practical application of 1DPCs. The mechanical stability was examined by continuous sonication of the Si substrate coated with the LDH/TiO2 film in water containing detergent. After sonication (100 W) for 1 h, the optical properties remained unchanged (Fig. S5, ESI†). Furthermore, no delamination or peeling occurred on cross-cutting the surface, indicating the strong adherence of the film to the substrate (Fig. S6, ESI†). The high durability and strong adhesion of the LDH/TiO2 film to the substrate should guarantee the long-term application of the 1DPCs under practical conditions.
In summary, 1DPCs have been fabricated by alternate deposition of TiO2 and LDH nanoparticles on a Si substrate. The optical response of the resulting LDH-based 1DPCs can be tailored by two approaches: (i) a tunable 1DPC whose color covered the whole visible range was obtained by changing the thickness of the TiO2 or LDH slabs; (ii) a reversible shift of the photonic stop band was achieved by tuning the refractive index of the LDH stacks via inducing the reversible phase transformation between LDHs and MMOs in a calcination–rehydration cycle. It is expected that such LDH/TiO2 1DPCs with high stability as well as sensitivity to external stimuli will find applications in intelligent optical sensing and smart windows. Further investigation of 1DPCs containing LDHs as an “active layer” are in progress, with the purpose of detecting and discriminating specific chemical/biological molecules.
This work was supported by the National Natural Science Foundation of China, the 863 Program (Grant No.: 2009AA064201), and the Fundamental Research Funds for the Central Universities (Grant No.: ZY1215).
References
- (a) M. S. Yoon, K. H. Ahn, R. W. Cheung, H. Sohn, J. R. Link, F. Cunin and M. J. Sailor, Chem. Commun., 2003,(6), 680 RSC; (b) J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829 CrossRef CAS.
- (a) S. Kubo, Z. Gu, K. Takahashi, A. Fujishima, H. Segawa and O. Sato, J. Am. Chem. Soc., 2004, 126, 8314 CrossRef CAS; (b) B. V. Lotsch and G. A. Ozin, ACS Nano, 2008, 2, 2065 CrossRef CAS; (c) F. M. Hinterholzinger, A. Ranft, J. M. Feckl, B. Rühle, T. Bein and B. V. Lotsch, J. Mater. Chem., 2012, 22, 10356 RSC.
- (a) O. Sánchez-Sobrado, G. Lozano, M. E. Calvo, A. Sánchez-Lglesias, L. M. Liz-Marzán and H. Míguez, Adv. Mater., 2011, 23, 2108 CrossRef; (b) J. Ge and Y. Yin, Angew. Chem., Int. Ed., 2011, 50, 1492 CrossRef CAS.
- (a) C. Chen, Y. Zhu, H. Bao, J. Shen, H. Jiang, L. Peng, X. Yang, C. Li and G. Chen, Chem. Commun., 2011, 47, 5530 RSC; (b) M. J. Sweetmana and N. H. Voelcker, RSC Adv., 2012, 2, 4620 RSC.
- A. C. Arsenault, D. P. Puzzo, I. Manners and G. A. Ozin, Nat. Photonics, 2007, 1, 468 CrossRef CAS.
- T. J. Alfrey, E. F. Gurnee and W. J. Schrenk, Polym. Eng. Sci., 1969, 9, 400 CAS.
- M. E. Calvo, S. Colodrero, T. C. Rojas, J. A. Anta, M. Ocaña and H. Míguez, Adv. Funct. Mater., 2008, 18, 2708 CrossRef CAS.
- Z. Wu, D. Lee, M. F. Rubner and R. E. Cohen, Small, 2007, 3, 1445 CrossRef CAS.
- M. E. Calvo, O. S. Sobrado, G. Lozano and H. Míguez, J. Mater. Chem., 2009, 19, 3144 RSC.
- S. Y. Choi, M. Mamak, G. Freymann, N. Chopra and G. A. Ozin, Nano Lett., 2006, 6, 2456 CrossRef CAS.
- W. Hong, X. Hu, B. Zhao, F. Zhang and D. Zhang, Adv. Mater., 2010, 22, 5043 CrossRef CAS.
- (a) S. Mitchell, I. R. Baxendale and W. Jones, Green Chem., 2008, 10, 629 RSC; (b) E. Géraud, V. Prévot, J. Ghanbaja and F. Leroux, Chem. Mater., 2006, 18, 238 CrossRef; (c) D.-Y. Wang, A. Das, A. Leuteritz, R. N. Mahaling, D. Jehnichen, U. Wagenknecht and G. Heinrich, RSC Adv., 2012, 2, 3927 RSC.
- (a) Z. P. Xu and P. S. Braterman, J. Phys. Chem. C, 2007, 111, 4021 CrossRef CAS; (b) J. A. Gursky, S. D. Blough, C. Luna, C. Gomez, A. N. Luevano and E. A. Gardner, J. Am. Chem. Soc., 2006, 128, 8376 CrossRef CAS.
- (a) L. Desigaux, M. B. Belkacem, P. Richard, J. Cellier, P. Léone, L. Cario, F. Leroux, C. Taviot-Guého and B. Pitard, Nano Lett., 2006, 6, 199 CrossRef CAS; (b) C. Coelho, M. Hennous, V. Verney and F. Leroux, RSC Adv., 2012, 2, 5430 RSC.
- (a) Y.-S. Yoon, S.-H. Byeon and I. S. Lee, Adv. Mater., 2010, 22, 3272 CrossRef CAS; (b) J. L. Gunjakar, T. W. Kim, H. N. Kim, I. Y. Kim and S.-J. Hwang, J. Am. Chem. Soc., 2011, 133, 14998 CrossRef CAS; (c) H. Yao, H. Fang, Z. Tan, L. Wu and S. Yu, Angew. Chem., Int. Ed., 2010, 49, 2140 CrossRef CAS.
- (a) G. R. Williams, A. J. Norquist and D. O'Hare, Chem. Commun., 2003,(15), 1816 RSC; (b) J. Han, Y. Dou, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2010, 49, 2171 CrossRef CAS; (c) J. Rocha, M. del Arco, V. Rives and M. A. Ulibarri, J. Mater. Chem., 1999, 9, 2499 RSC; (d) F. Millange, R. I. Walton and D. O'Hare, J. Mater. Chem., 2000, 10, 1713 RSC.
- (a) M. Born and E. Wolf, Principles of Optics, Pergamon, Oxford, UK, 1980 Search PubMed; (b) D. P. Puzzo, L. D. Bonifacio, J. Oreopoulos, C. M. Yip, I. Manners and G. A. Ozin, J. Mater. Chem., 2009, 19, 3500 RSC.
- O. D. Pavel, R. Birjega, M. Che, G. Costentin, E. Angelescu and S. Serban, Catal. Commun., 2008, 9, 1974 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, characterization of LDH and TiO2 precursors, AFM images, XRD patterns, reflectance spectra and stability investigation. See DOI: 10.1039/c2ra20790g |
| This journal is © The Royal Society of Chemistry 2012 |
