The Au(III)-catalyzed coupling reactions between alcohols and N-heterocycles via C–H bond activation†
Honglai
Jiang
a,
Jin
Xie
a,
Aijun
Lin
a,
Yixiang
Cheng
a and
Chengjian
Zhu
*ab
aState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P. R. China. E-mail: cjzhu@nju.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, P. R. China
First published on 17th September 2012
Abstract
A novel and highly efficient dehydrogenative C–C coupling between sp3 C–H and sp2 C–H bonds catalyzed by gold has been developed using environmentally benign tert-butyl hydroperoxide as oxidant. This new methodology provides a facile access to acylate N-heterocycles.
The construction of the C–C bond is the most essential subject in chemistry since they can provide key steps in building more complex products from readily available materials. Lately, as C–H bonds are unique in organic molecules, the activation of the C–H bond for C–C coupling without substrate prefunctionalization has become a promising strategy.1 More recently, homogeneous gold-catalyzed C–C bond formation has emerged as a very exciting area of research due to its powerful advantages for the synthesis of intricate scaffolds.2 The groups of Tse and Nevado reported the gold-catalyzed oxidative homocoupling of simple arenes and oxidative ethynylation of arenes with terminal alkynes using PhI(OAc)2 as an oxidant.3 During our group research, we also demonstrated a gold-catalyzed oxidative C–C coupling of Csp3–H bonds under TBHP or air conditions.4 However, all the above gold-catalyzed work focused on the sp3 C–H/sp3 C–H, sp C–H/sp2 C–H and sp2 C–H/sp2 C–H oxidative coupling. To the best of our knowledge, the gold-catalyzed oxidative C–C coupling from sp3 C–H and sp2 C–H bonds are rarely reported (Scheme 1). Moreover, activation of aromatic C–H bonds followed by generating a new C–C bond constitutes a conceptually promising methodology since it avoids the otherwise prefunctionalization of the aromatic counterpart.5
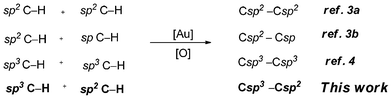 | ||
| Scheme 1 Au-catalyzed oxidative coupling reactions from C–H bonds. | ||
Recently, the activation of C–H bonds adjacent to oxygen has intrigued much attention because of the feasibility of formation a new C–C bond and the introduction of an oxygen-containing functional group into the organic molecule at the same time.6 However, the direct and selective activation of α C–H of alcohols remains a great challenge to chemists. Traditionally, alcohols are rarely employed as acylating agents since they are inclined to undergo energetically more favorable alkoxylation and dehydrogenative process followed by cleavage of the C–O bond (C–O bond dissociation energy = 85 to 91 kcal mol−1).7 The pioneering research in this area about the hydroxymethylation and hydroxyethylation of N-heterocycles were explored by Minisci,8 in the presence of peroxides and equivalents of acid with moderate and low yields respectively. Recently, Li and co-workers reported a palladium-catalyzed C–C coupling reactions of alcohols and N-heterocycles using DCP as an oxidant.9 At the same time, Wang's group demonstrated C2 alkylation of azoles with alcohols and ethers through dehydrogenative cross-coupling under metal-free conditions.10 Herein we became interested in the gold-catalyzed oxidative C–C coupling of N-heterocycles with various alcohols as Csp3–H coupling partners (Scheme 2).
 | ||
| Scheme 2 Au-catalyzed the dehydrogenative coupling between alcohols and isoquinoline. | ||
In our initial study, the oxidative C–C coupling reaction of lepidine with ethanol was chosen as the model reaction. To our delight, the reaction could occur under the catalysis of 4a (5 mol%) at 120 °C under air with TBHP as oxidant, affording the product in 63% yield. Surprisingly, unlike the previous report, the acylation product of the lepidine rather than the alcohol was observed.9,11 Then, different gold catalysts and other metal catalysts were tested. Among the Au(III) catalysts screened, 4c showed the best catalytic activity (Table 1, entries 1–3). Meanwhile, the utilization of Ph3PAuCl or AuCl instead of Au(III) catalysts also resulted in a low yield (Table 1, entries 5,6). In addition, when other metal catalysts such as CuI, FeIII and NiII were examined instead of 4c, the reaction proceeded very poorly (Table 1, entries 7–9). which indicated that gold catalyst 4c is necessary and plays an important role in the C–H activation process. Either trace amounts or no product was detected when the other oxidants were employed (Table 1, entries 11–13). Notably, the yields decreased under oxidative reaction conditions when the amount of oxidant (TBHP) and catalyst 4c loading was reduced (Table 1, entries 14–16). Consequently, the optimal reaction conditions included catalysis by 5 mol% of 4c at 120 °C under air in the presence of TBHP as oxidant.12
| Entry | Cat (mol%) | Oxidant | Yield (%)b |
|---|---|---|---|
a Conditions: 1a (0.5 mmol), ethanol 2a (2 mL), catalyst 4 (5 mol%), oxidant (4.5 equiv), 120 °C, 24 h;
b isolated yield.

|
|||
| 1 | 4a(5) | TBHP(4.5) | 63 |
| 2 | 4b(5) | TBHP(4.5) | 46 |
| 3 | 4c(5) | TBHP(4.5) | 73 |
| 4 | 4d(5) | TBHP(4.5) | 65 |
| 5 | 4e(5) | TBHP(4.5) | 59 |
| 6 | 4f(5) | TBHP(4.5) | 58 |
| 7 | FeCl3(5) | TBHP(4.5 | n.d |
| 8 | CuI(5) | TBHP(4.5) | n.d |
| 9 | NiCl2(5) | TBHP(4.5) | 23 |
| 10 | none | TBHP(4.5) | 0 |
| 11 | 4c(5) | NaClO(4.5) | 0 |
| 12 | 4c(5) | TEMPO(4.5) | 0 |
| 13 | 4c(5) | mCPBA(4.5) | trace |
| 14 | 4c(5) | TBHP(3.5) | 53 |
| 15 | 4c(5) | TBHP(2.0) | 26 |
| 16 | 4c(2.5) | TBHP(4.5) | 65 |
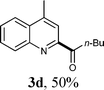
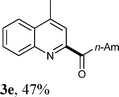
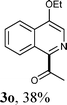
Under the optimized reaction conditions, the scope of the alcohols in the direct oxidative coupling of lepidine (1a) was investigated. The results are shown in Table 2. As can be seen from Table 2, the reactions of lepidine with aliphatic alcohols, such as ethanol, n-propanol, n-butanol, n-pentanol and n-heptanol, produced the corresponding dehydrogenative cross-coupling products (3a–f) in moderate to good yields. It is important to point out that increasing the chain length of the aliphatic primary alcohols showed a little influence on the yield of the corresponding products. Furthermore, a variety of nitrogen containing heterocycles were also investigated. It was found that quinolines and isoquinolines were effective substrates for the reaction, affording the desired product with satisfactory yields. Significantly, the electron-withdrawing groups on the aromatic rings usually gave the products (3i–l) in higher yields (53–73%), than those with electron-donating groups (3h and 3o). It should be noted that the acylation reactions proceeded with high regioselectivity. For example, only one isomer can be isolated when the N-heterocycles had alternative sp2 C–H bonds adjacent to an N-heteroatom (3g, 3k).
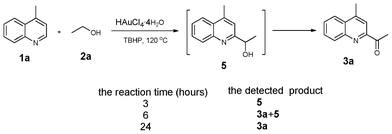
To gain insight into the reaction mechanism, the ESI-MS was employed to determine whether the final product 3a was transferred from the alcohol intermediate 5 (Fig. 1). Intriguingly, the intermediate 5 was detected by ESI-MS when the reaction proceeded for three hours, and no acylation product 3a was observed. Six hours later, both the alcohol intermediate 5 and the final product 3a could be found. Moreover, only the desired product 3a was obtained when the reaction finished, and the alcohol intermediate 5 disappeared completely.13 These results indicated that the reaction proceeded through the activation of α sp3 C–H of alcohols. When the radical inhibitors such as BHT (2,6-di-tert-butyl-4-methylphenol) and TEMPO (2,2,6,6-tetramethylpiperidine-N-oxyl) were added into the reaction of ethanol 1a and lepidine 2a, no acylation product was detected.
 | ||
| Fig. 1 The monitored results of the reaction of 1a and 2a by ESI-Ms. | ||
From these results we believe that the reaction proceeded through the activation α C–H of alcohols by a radical mechanism14 similar to Minisci reaction.8 Initially, a hydroxyl radical and an alkoxyl radical are generated under heating from a homolytic cleavage of tert-butyl hydroperoxide (Scheme 3). The generated free radicals 6 subsequently undergo a hydrogen atom abstraction from the α-position sp3 C–H of ethanol 2a, forming the corresponding free radical 7. It is probable that this radical 7 reacts with the gold-activated lepidine 1a to form radical 9, which is rearomatized by radical initiator, delivering the detected alcohol intermediate 5. Then, the alcohol intermediate 5 is further turned into the acylation product 3a through a gold-catalyzed oxidation process with TBHP.15 Although the role of gold in the reaction is not very clear, we believe it plays a very important role in the process, as a trace amount of desired product or no product 3a could be isolated when other metal catalysts was used instead of 4c (Table 1, entries 14–16). It is possible that gold may be participating in the generation of the radical intermediate 7 and oxidized the alcohol intermediate 5 into the final product 3a.
In summary, the first gold-catalyzed C–C bond formation based on the direct oxidative Csp3–H/Csp2–H coupling reactions involving N-heterocycles and alcohols has been developed. This novel methodology provides a facile access to direct acylation of the N-heterocycles. A detailed mechanistic study and further investigation on the application of this kind of oxidant under gold are currently underway in our laboratory.
 | ||
| Scheme 3 The possible reaction mechanism. | ||
This work is supportrd by National Natural Science Foundation of China (20832001, 20972065, 21074054, 21172106) and National Basic Research Program of China (2010CB923303) for their financial support.
References
- For selected reviews of C–C coupling from C–H bonds, see: (a) Z. Li, D. Bohle and C. J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS; (b) C. J. Li and Z. Li, Pure Appl. Chem., 2006, 78, 935 CrossRef CAS; (c) C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS; (d) W. J. Yoo and C. J. Li, Top. Curr. Chem., 2010, 292, 281; (e) C. J. Scheuermann, Chem.–Asian J., 2010, 5, 436 CrossRef CAS; (f) S. L. You and J. B. Xia, Top. Curr. Chem., 2010, 292, 165 CAS; (g) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS; (h) C. Liu, H. Zhang, W. Shi and A. W. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS; (i) W. Shi, C. Liu and A. W. Lei, Chem. Soc. Rev., 2011, 40, 2761 RSC; (j) S. Gaillard, C. S. Cazin and A. P. Nolan, Acc. Chem. Res., 2012, 45, 778 CrossRef CAS; (k) Z. Z. Shi, C. Zhang, C. H. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC.
- (a) H.A. Wegner and M. Auzias, Angew. Chem., Int. Ed., 2011, 50, 8236 CAS; (b) M. N. Hopkinson, D. G. Antony and V. Gouverneur, Chem.–Eur. J., 2011, 17, 8248 CrossRef CAS; (c) J. Xiao and X. W. Li, Angew. Chem., Int. Ed., 2011, 50, 7226 CrossRef CAS.
- (a) A. Kar, N. Mangu, H. M. Kaiser and M. K. Tse, J. Organomet. Chem., 2009, 694, 524 CrossRef CAS; (b) T. Haro and C. Nevado, J. Am. Chem. Soc., 2010, 132, 1512 CrossRef CAS.
- (a) J. Xie, H. M. Li, J. C. Zhou, Y. X. Cheng and C. J. Zhu, Angew. Chem., Int. Ed., 2012, 51, 1252 CrossRef CAS.
- C. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura and Y. Fujiwara, Science, 2000, 287, 1992 CrossRef CAS.
- (a) R. L. Patman, J. F. Bower, I. S. Kim and M. J. Krische, Aldrichimica Acta., 2008, 41, 95 CAS; (b) J. F. Bower, I. S. Kim, R. L. Patman and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 34 CrossRef CAS; (c) V. M. Williams, J. C. Leung, R. L. Patman and M. J. Krische, Tetrahedron, 2009, 65, 5024 CrossRef CAS; (d) J. R. Zbieg, E. Yamaguchi, E. L. McInturff and M. J. Krische, Science, 2012, 336, 324 Search PubMed.
- M. B. Smith and J. March, March's Advanced Organic ChemistryWiley, New York, 2001 Search PubMed.
- (a) F. Minisci, Synthesis, 1973, 1 CrossRef CAS; (b) F. Minisci, A. Vismara, F. Fontana, G. Morini and M. Serravalle, J. Org. Chem., 1986, 51, 476 CrossRef CAS; (c) F. Minisci, A. Cetterio, A. Vismara and C. Giordano, Tetrahedron Lett., 1985, 26, 617; (d) F. Minisci, A. Citterio, E. Vismara and C. Giordano, Tetrahedron, 1985, 41, 4157 CrossRef CAS.
- C. A. Correia, L. Yang and C. J. Li, Org. Lett., 2011, 13, 4581 CrossRef CAS.
- T. He, L. Yu, L. Zhang, L. Wang and M. Wang, Org. Lett., 2011, 13, 5016 CrossRef CAS.
- (a) T. Caronna, G. Fronza, F. Minisci and O. Porta, J. Chem. Soc., Perkin Trans. 2, 1972, 2035 RSC; (b) Y. Yuan, D. T. Chen and X. W. Wang, Adv. Synth. Catal., 2011, 353, 3373 CrossRef CAS; (c) F. H. Xiao, Q. Shuai, F. Zhao, O. Baslé, G. J. Deng and C. J. Li, Org. Lett., 2011, 13, 1614 CrossRef CAS.
- The procedure for the synthesis of 3a: HAuCl4·4H2O (0.025 mmol), TBHP (2.25 mmol) were placed in a dry sealable tube. To this, dried ethanol 2a (2.5 mL) and lepidine (0.5 mmol) 1a were added. The tube was sealed, and stirred for 24 h at 120 °C. The reaction mixture was cooled to room temperature and flushed through a short column of silica gel with ethyl acetate. The solvent was removed under vacuum, and product 3a was isolated by flash column chromatography.
- The intermediate was detected by MS when the reaction was stirred three hours, which further turned into product as the reaction proceeded on. For details please see the ESI†.
- A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS.
- (a) B. T. Guan, D. Xing, G. X. Cai, X. B. Wan, N. Yu, Z. Fang, L. P. Yang and Z. J. Shi, J. Am. Chem. Soc., 2005, 127, 18004 CrossRef CAS; (b) H. R. Lia, B. T. Guana, W. J. Wang, D. Xing, Z. Fang, X. B. Wan, L. P. Yang and Z. J. Shi, Tetrahedron, 2007, 63, 8430 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details and the characterization data for the products. See DOI: 10.1039/c2ra22120a |
| This journal is © The Royal Society of Chemistry 2012 |