Enhanced visible-light photocatalytic degradation of methyl orange by BiPO4–CdS composites synthesized using a microwave-assisted method
Tian
Lv
,
Likun
Pan
*,
Xinjuan
Liu
and
Zhuo
Sun
Engineering Research Center for Nanophotonics & Advanced Instrument, Ministry of Education, Department of Physics, East China Normal University, Shanghai 200062, China. E-mail: lkpan@phy.ecnu.edu.cn; Fax: +86 21 62234321; Tel: +86 21 62234132
First published on 24th October 2012
Abstract
BiPO4–CdS composites consisting of BiPO4 nanorods and CdS nanoparticles were successfully synthesized via a microwave-assisted reaction. The photocatalytic performance of the BiPO4–CdS composites, with different proportions of CdS, in the degradation of methyl orange under visible light irradiation was investigated. The results show that the BiPO4–CdS composite with 30.1 wt% CdS achieves a better performance with a maximum degradation rate of 98.1% as compared with pure BiPO4 due to the increase of specific surface area for more adsorbed MO, the enhancement of light absorption and the reduction of carrier recombination.
Semiconductor oxide photocatalysis has attracted much attention for water treatment due to its good destruction ability of pollutants and broad compound applicability.1–4 Among various semiconductor oxide photocatalysts, TiO2 has been used widely for environmental applications due to its intriguing optical and electric properties, low cost, nontoxicity, and ease of availability.5–7 However, the photocatalytic activity of TiO2 is not high enough to meet the need of industrial usages due to the rapid recombination of photogenerated electrons and holes, which causes high running costs.8
Recently, BiPO4 has been recognized as an excellent photocatalyst with superior photocatalytic activity to P25 (the commercial TiO2) in the UV region when used for the destruction of dyes and pollutants.8–12 Liu et al. obtained BiPO4 nanorods via a hydrothermal method and the BiPO4 nanorods showed a high efficiency in the photocatalytic degradation of methylene blue (MB) under UV light irradiation.9 Pan and Zhu synthesized BiPO4 nanocrystals by a high-temperature hydrolysis reaction and the BiPO4 nanocrystals exhibited high activity in the degradation of MB under UV light irradiation.12 However, similar to TiO2, the wide band gap of BiPO4 (∼3.85 eV) limits its photocatalytic property to the UV region, which only occupies 3–5% of the total solar spectrum.13,14 To improve the sunlight photocatalytic efficiency, many promising studies have reported the coupling of TiO2 with a narrow band gap semiconductor material, typically CdS (2.4 eV), to extend the absorption to the visible light range.15–19 In the meantime, the photoexcited electrons in CdS can be efficiently injected into TiO2 because the conduction band (CB) level of CdS is higher than that of TiO2.20,21 Similarly, the combination of BiPO4 and CdS should be an ideal system to achieve good photocatalytic performance under visible light irradiation. Firstly, CdS can extend the optical response to the visible light range. Secondly, as shown in Fig. 1, on the basis of the relevant band positions of BiPO4 and CdS (CB: −4.07 eV and −3.98 eV vs. vacuum),8,22 photo-induced electrons easily transfer from CdS CB to BiPO4 CB, which could efficiently separate the photo-induced electrons and hinder the charge recombination in electron-transfer processes. Unfortunately, there has been no report on the synthesis and investigation of BiPO4–CdS composites for photocatalysis until now.
 | ||
| Fig. 1 Schematic diagram of energy levels of BiPO4 and CdS. CB and VB are the conduction band and valence band, respectively. | ||
In this work, a simple synthesis of BiPO4–CdS composites was carried out through a microwave-assisted reaction for the first time. Microwave irradiation heats the reactant to a high temperature in a short time by transferring energy selectively to microwave absorbing polar solvents.23,24 Thus it can facilitate mass production in a short time span with little energy consumption25,26 and form an intimate contact between the components,27,28 which is crucial for the formation of electronic interaction and interelectron transfer at the interface. The as-synthesized BiPO4–CdS composites exhibit an enhanced photocatalytic performance in the degradation of methyl orange (MO) under visible light irradiation as compared with pure BiPO4.
1 mmol Bi(NO3)3·5H2O and 1 mmol Na3PO4·12H2O were added in 20 mL distilled water and magnetically stirred to form a homogeneous solution at room temperature. The solution was then put into an automated focused microwave system (Explorer-48, CEM Co.) and treated at 150 °C with a microwave irradiation power of 100 W for 15 min. The as-synthesized BiPO4 was isolated by filtration, washed several times with distilled water, and finally dried in a vacuum oven at 60 °C for 24 h.
Different amounts of CdCl2·2.5H2O and Na2S·9H2O (Sinopharm Chemical Reagents Co. Ltd.) were added into the as-prepared BiPO4 suspensions and then the solutions were sonicated for 30 min to produce uniform dispersions and were then treated at 100 °C with a microwave irradiation power of 100 W for 5 min. The as-synthesized BiPO4–CdS samples with 8.9, 16.4, 30.1 and 45.5 wt% CdS, named as BC-1, BC-2, BC-3 and BC-4, were isolated by filtration, washed several times with distilled water, and finally dried in a vacuum oven at 60 °C for 24 h.
The surface morphology, structure and composition of the samples were characterized by field-emission scanning electron microscopy (FESEM, Hitachi S-4800), high-resolution transmission electron microscope (HRTEM, JEOL-2010), energy dispersive X-ray spectroscopy (EDS, JEM-2100) and X-ray diffraction spectroscopy (XRD, Holland Panalytical PRO PW3040/60) with Cu-Kα radiation (V = 30 kV, I = 25 mA), respectively. The Brunauer–Emmett–Teller (BET) specific surface areas of the samples were evaluated on the basis of nitrogen adsorption isotherms measured at 77 K using a ASAP 2010 apparatus (Micromeritics, U.S.A). All the samples were degassed at 160 °C before nitrogen adsorption measurements. The BET surface area was determined using the adsorption data in the relative pressure (P/P0) range of 0.025–0.999. The UV-vis absorption spectra were recorded using a Hitachi U-3900 UV-vis spectrophotometer.
The photocatalytic performance of the as-prepared samples was evaluated by photocatalytic degradation of MO under visible light irradiation. 150 mg BiPO4–CdS powders were added into 100 mL MO aqueous solutions (10 mg L−1). The mixed suspension was continuously stirred for 0.5 h in the dark to reach an adsorption–desorption equilibrium. Under ambient conditions and stirring, the mixed suspension was exposed to the visible irradiation produced by a 400 W metal halogen lamp with cut off filter (λ > 400 nm). At certain time intervals, 2 mL of the mixed suspension was extracted and centrifugated to remove the photocatalyst. The degradation process was monitored by measuring the absorption of MO in the filtrate at 463 nm using an UV-vis absorption spectrometer.
The FESEM images of BiPO4, BC-1, BC-2, BC-3 and BC-4 are shown in Fig. 2(a)–(e). The BiPO4 nanorod is 600–700 nm in length and 100–200 nm in width. It can be observed from Fig. 2(b) that a small amount of CdS has been distributed on the surface of BiPO4 as compared with bare BiPO4 nanorods [Fig. 2(a)]. A more compact surface is observed from Fig. 2(c)–(e), indicating that more CdS nanoparticles have been deposited on the surface of BiPO4. The existence of CdS and BiPO4 in the composite has been proved by the peaks of Cd, S, Bi, P and O in EDS data [Fig. 2(f)]. The BET specific surface areas of BiPO4, BC-1, BC-2, BC-3 and BC-4 are found to be 4.1, 35.9, 50.0, 56.3 and 47.0 m2 g−1, respectively, indicating that the increase of CdS can supply more surface sites to accommodate more adsorbed MO, which is beneficial to the photocatalytic degradation of MO. However, excessive loading of CdS nanoparticles may cause their aggregation, as indicated from the morphology of the BC-4 sample in Fig. 2(e), and thus decreases the specific surface area.
 | ||
| Fig. 2 FESEM images of (a) BiPO4 (b) BC-1, (c) BC-2, (d) BC-3 and (e) BC-4; (f) EDS spectrum of BC-3. | ||
The low-magnification and high-magnification HRTEM images of BC-3 are shown in Fig. 3(a) and (b). It is clearly found from Fig. 3(a) that the surface of the BiPO4 nanorods is decorated with CdS nanoparticles. The large crystallites are identified to be BiPO4 nanorods, as shown in Fig. 3(b). The lattice spacing measured for the crystalline plane is 0.442 nm, corresponding to the (101) plane of the hexagonal phase BiPO4 (JCPDS#45-1370).11 Around the BiPO4 crystallite edge, fine crystallites are observed. The crystallites connecting to the BiPO4 have lattice fringes of 0.335 nm, which is ascribed to the (111) plane of CdS (JCPDS#80-0019). The HRTEM image also indicates that CdS, with a size of about 5 nm is presented in a crystalline structure.
 | ||
| Fig. 3 (a) Low-magnification and (b) high-magnification HRTEM images of BC-3. | ||
The XRD patterns of BiPO4, BC-1, BC-2, BC-3 and BC-4 are shown in Fig. 4. Compared with the XRD pattern of BiPO4, in which several peaks correspond to hexagonal and monoclinic phases of BiPO4 (JCPDS#45-1370, JCPDS#80-0209), three main peaks corresponding to the (111), (220) and (311) planes of CdS (JCPDS#80-0019) appear in other XRD patterns. This further confirms the existence of CdS on BiPO4. The signals corresponding to CdS indicate the CdS crystallites are mainly present as a cubic structure and their relative intensity increases with the increase of CdS amount.
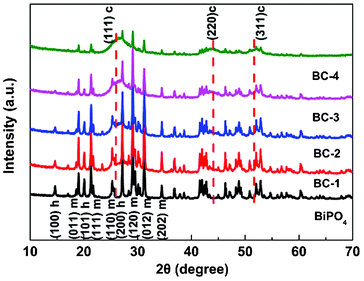 | ||
| Fig. 4 XRD patterns of BiPO4, BC-1, BC-2, BC-3 and BC-4. c –cubic CdS, h – hexagonal and m – monoclinic BiPO4. | ||
Fig. 5(a) shows the UV-vis absorption spectra of BiPO4, BC-1, BC-2, BC-3 and BC-4. Compared with the spectrum of BiPO4, there is an obvious absorption peak near 500 nm for BiPO4–CdS composites, which is ascribed to the contribution from CdS. The band gap of the CdS QDs, corresponding to the absorption edge, is about 2.38 eV. The gradual increase of absorbance from BC-1 to BC-4 shows that more CdS has been attached onto the surface of BiPO4, which is similar to those reported in TiO2–CdS composites.29 This result is consistent with the FESEM observation. The photocatalytic degradation of MO under visible light irradiation was used to evaluate the photocatalytic performance of BiPO4, BC-1, BC-2, BC-3 and BC-4, as shown in Fig. 5(b). It was observed that MO is hardly degraded under visible light in the absence of the photocatalyst. Only 21.1% MO can be photodegraded by pure BiPO4 under visible light in 5 h. However, all BiPO4–CdS composites exhibit a better photocatalytic performance than pure BiPO4. When CdS is introduced into BiPO4, the degradation rate is increased to 85.1% and 93.4% for BC-1 and BC-2 and reaches a maximum value of 98.1% for BC-3, which is due to the increased specific surface area that supplies more surface sites to accommodate more adsorbed MO, the enhanced light absorption and the stepwise structure of the energy levels constructed in the BiPO4–CdS composite (Fig. 1). However, when the loading amount of CdS is further increased, the photocatalytic performance deteriorates. This is ascribed to the following reasons: (i) it will be more difficult to transport an electron from CdS into BiPO4 at a high CdS loading because of the increase in the average distance and electron injection time from CdS to BiPO4;30 (ii) more CdS content would reduce the specific surface area of BiPO4 to adsorb MO;31 (iii) charge carriers dissipate by trapping when the loading amount of CdS comes up to a certain level.32
 | ||
| Fig. 5 (a) UV-vis absorption spectra of BiPO4, BC-1, BC-2, BC-3 and BC-4; (b) photocatalytic degradation of MO by BiPO4, BC-1, BC-2, BC-3 and BC-4 under visible light irradiation. | ||
The specific surface area of the photocatalyst plays an important role in photocatalytic degradation.33 The larger the surface area of the photocatalyst, the more reaction sites there are, which is in favor of the photocatalytic activity.34 In order to rule out the effect of specific surface areas on the photocatalytic activities, the photocatalytic reaction rates should be normalized by the specific surface areas.35–37 Zhang et al.38 investigated the reaction rate constants (k) and k′ (k values normalized by the surface areas) of BiOI in the MO degradation and found that the order for the k′ was the same as that of the original k. Fig. 6 shows the linear fitting using the pseudo-first-order kinetic equations to the experimental data for BiPO4, BC-1, BC-2, BC-3 and BC-4. The values of k can be obtained directly from the fitted straight-line plots of ln(Ci/C0) versus reaction time, which follow the order: BC-3 (0.0134 min−1) > BC-4 (0.0105 min−1) > BC-2 (0.0098 min−1) > BC-1 (0.0068 min−1) > BiPO4 (0.00067 min−1). The k′ (normalized k) values are 0.00016 g min−1 m−2, 0.00019 g min−1 m−2, 0.0002 g min−1 m−2, 0.00024 g min−1 m−2, and 0.00022 g min−1 m−2 for BiPO4, BC-1, BC-2, BC-3 and BC-4, respectively. The results indicate that the order of the normalized rates is the same as that of the original rates, which is similar to that reported in the literature.38 The enhanced photocatalytic activity with the increase of CdS content should be ascribed to the enhanced light absorption. BC-3 exhibits the best photocatalytic activity. The photocatalytic activity of BC-4 is worse than that of BC-3 due to the more difficult electron transport and the charge carriers dissipate by trapping when the loading amount of CdS comes up to a certain level.30,32
 | ||
| Fig. 6 Photocatalytic reaction kinetics of MO with reaction time. | ||
In summary, we have demonstrated a simple, rapid and effective microwave-assisted method to synthesize BiPO4–CdS composites for the degradation of MO. The results of the photocatalytic experiments indicate that (i) the BiPO4–CdS composites exhibit better photocatalytic performances than pure BiPO4 under visible light irradiation; (ii) the photocatalytic performances of the BiPO4–CdS composites is dependent on the proportion of CdS, and the composite with 30.1 wt% CdS achieves the highest degradation rate of 98.1% for the degradation of MO; (iii) the enhanced photocatalytic performance is ascribed to the increase of the specific surface area for more adsorbed MO, the enhancement of light absorption and the reduction of carrier recombination.
References
- G. L. Xiang, D. Wu, J. He and X. Wang, Chem. Commun., 2011, 47, 11456 RSC.
- Z. G. Xiong, L. L. Zhang, J. Z. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099 RSC.
- P. Ma, Y. Wu, Z. Fu and W. Wang, J. Alloys Compd., 2011, 509, 3576 CrossRef CAS.
- D. Zhang, X. Liu and X. Wang, J. Alloys Compd., 2011, 509, 4972 CrossRef CAS.
- M. Y. Guo, M. K. Fung, F. Fang, X. Y. Chen, A. M. C. Ng, A. B. Djurisic and W. K. Chan, J. Alloys Compd., 2011, 509, 1328 CrossRef CAS.
- X. Zeng, Y. X. Gan, E. Clark and L. Su, J. Alloys Compd., 2011, 509, L221 CrossRef CAS.
- J. Zhang, Y. P. Zhang, Y. K. Lei and C. X. Pan, Catal. Sci. Technol., 2011, 1, 273 CAS.
- C. S. Pan and Y. F. Zhu, Environ. Sci. Technol., 2010, 44, 5570 CrossRef CAS.
- Y. F. Liu, X. G. Ma, X. Yi and Y. F. Zhu, Acta Phys. Chim. Sin., 2012, 28, 654 Search PubMed.
- C. S. Pan, J. Xu, Y. Chen and Y. F. Zhu, Appl. Catal., B, 2012, 115–116, 314 CrossRef CAS.
- G. F. Li, Y. Ding, Y. F. Zhang, Z. Lu, H. Z. Sun and R. Chen, J. Colloid Interface Sci., 2011, 363, 497 CrossRef CAS.
- C. S. Pan and Y. F. Zhu, J. Mater. Chem., 2011, 21, 4235 RSC.
- S. U. Khan, M. Al-Shahry and W. B. Jr, Science, 2002, 297, 2243 CrossRef CAS.
- Z. G. Zou, J. H. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625 CrossRef CAS.
- Q. Li, T. Kako and J. Ye, J. Mater. Chem., 2010, 20, 10187 RSC.
- Y. Xie, G. Ali, S. H. Yoo and S. O. Cho, ACS Appl. Mater. Interfaces, 2010, 2, 2910 CAS.
- S. C. Hayden, N. K. Allam and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 14406 CrossRef CAS.
- J. Bai, J. Li, Y. Liu, B. Zhou and W. Cai, Appl. Catal., B, 2010, 95, 408 CrossRef CAS.
- A. H. Zyoud, N. Zaatar, I. Saadeddin, C. Ali, D. Park, G. Campet and H. S. Hilal, J. Hazard. Mater., 2010, 173, 318 CrossRef CAS.
- T. Lv, L. K. Pan, X. J. Liu, T. Lu, G. Zhu, Z. Sun and C. Q. Sun, Catal. Sci. Technol., 2012, 2, 754 CAS.
- G. Zhu, F. F. Su, T. Lv, L. K. Pan and Z. Sun, Nanoscale Res. Lett., 2010, 5, 1749 CrossRef CAS.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef.
- T. Lv, L. K. Pan, X. J. Liu, T. Lu, G. Zhu and Z. Sun, J. Alloys Compd., 2011, 509, 10086 CrossRef CAS.
- Y. C. Chen, S. L. Lo, H. H. Ou and C. H. Chen, Water Sci. Technol., 2011, 63, 550 CrossRef CAS.
- G. Zhu, L. K. Pan, T. Xu, Q. Zhao, B. Lu and Z. Sun, Nanoscale, 2011, 3, 2188 RSC.
- S. Horikoshi, H. Abe, K. Torigoe, M. Abe and N. Serpone, Nanoscale, 2010, 2, 1441 RSC.
- X. J. Liu, L. K. Pan, T. Lv, G. Zhu, Z. Sun and C. Q. Sun, Chem. Commun., 2011, 47, 11984 RSC.
- G. Zhu, L. K. Pan, T. Xu, Q. Zhao and Z. Sun, ACS Appl. Mater. Interfaces, 2011, 3, 1472 CAS.
- L. Wu, J. C. Yu and X. Z. Fu, J. Mol. Catal. A: Chem., 2006, 244, 25 CrossRef CAS.
- N. Guijarro, T. Lana-Villarreal, Q. Shen, T. Toyoda and R. Gomez, J. Phys. Chem. C, 2010, 114, 21928 CAS.
- L. Ge and J. Liu, Appl. Catal., B, 2011, 105, 289 CrossRef CAS.
- A. E. Raevskaya, A. L. Stroyuk, G. Ya. Grodzyuk, S. Ya. Kuchmii, V. M. Dzhagan, V. F. Plyusnin and V. P. Grivin, Theor. Exp. Chem., 2010, 46, 273 CrossRef CAS.
- M. Hermanek, R. Zboril, I. Medrik, J. Pechousek and C. Gregor, J. Am. Chem. Soc., 2007, 129, 10929 CrossRef CAS.
- V. Stengl and S. Bakardjieva, J. Phys. Chem. C, 2010, 114, 19308 CAS.
- G. Liu, Y. N. Zhao, C. H. Sun, F. Li, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2008, 47, 4516 CrossRef CAS.
- W. Li, Y. Bai, W. J. Liu, C. Liu, Z. H. Yang, X. Feng, X. H. Lu and K.Y. Chan, J. Mater. Chem., 2011, 21, 6718 RSC.
- Q. Sun and Y. M. Xu, J. Phys. Chem. C, 2010, 114, 18911 CAS.
- X. Zhang and L. Z. Zhang, J. Phys. Chem. C, 2010, 114, 18198 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
