Facile synthesis of uniform h-BN nanocrystals and their application as a catalyst support towards the selective oxidation of benzyl alcohol†
Liancheng
Wang
bc,
Liuliu
Shen
a,
Xiaohong
Xu
a,
Liqiang
Xu
*a and
Yitai
Qian
ab
aKey Laboratory of Colloid and Interface Chemistry(Shandong University), Ministry of Education, Jinan 250100, PR China. E-mail: xulq@sdu.edu.cn; Fax: +86-531-8836-6280; Tel: +86-531-8836-6280
bDepartment of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, People's Republic of China
cKey Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, 030001, Shanxi, People's Republic of China. E-mail: wanglc@sxicc.ac.cn
First published on 12th September 2012
Abstract
In this study, a high yield of uniform h-BN nanocrystals that are composed of two sets of exposed {102} and {002} planes has been prepared by using NaBH4, FeCl3 and NaN3 in a stainless steel autoclave at 750 °C. The cathodoluminescent (CL) properties of the samples indicates their potential application as optoelectronic materials in the ultraviolet region; they have also been used as a gold nanoparticle support for the relatively highly selective oxidation of benzyl alcohol to benzaldehyde (the conversion and selectivity were ∼30% and 98%, respectively).
1 Introduction
Hexagonal boron nitride (h-BN) is an important layered material that has wide potential applications owing to its high thermal stability, excellent chemical stability and good thermal conductivity.1 A near-band edge emission at 215 nm makes it a promising candidate for deep-blue and UV applications.2 Due to their shape-induced electrical and optical properties, the synthesis of h-BN nanopowders,3 especially those with special morphologies, such as 1D nanotubes (nanowires or nanofibers),4–6 2D nanosheets (or nanoplates),7–9 3D nanoflowers10,11 and hollow structures (nanocages, sphere and nanohorns12) has attracted a lot of research interest. It is well known that BN materials with high specific surface areas and sharp edges would provide BN with a high activity and improved properties.8,13 On the other hand, it has been pointed out that except for high specific surface areas of the nanomaterials, tuning the exposed facets of nanocrystals is an alternative and effective route to meet the increasing demands.14,15The nanocrystals (NCs) with intrinsic anisotropic structures, different exposed facets, especially with a high density of atomic steps and kinks, can endow NCs with higher activity, thus promoting their potential applications as highly efficient catalysts and special optical or gas sensing devices.16–18 For example, Co3O4 nanorods with predominantly exposed (110) planes display high catalytic oxidation ability toward CO even at −77 °C.15 Since the synthesis of tetrahexahedral platinum NCs (enclosed by 24 high-index facets, i.e. {730}) in 2007,18a unprecedented efforts have been devoted to the synthesis of micro- and NCs with different geometries and exposed facets in recent years, for example, trisoctahedral gold NCs with exposed {221} planes18b and tetrakaidecahedral Fe2O3 NCs with exposed {012}16 planes. These reports mainly deal with noble metals (cubic crystal systems) and limited oxides. Whereas, the binary nitrides, especially layered materials with strong in-plane bonds and weak coupling between layers, have been little investigated so far.
Due to the anisotropic structures and shape determining the surface atomic arrangement and coordination, BN NCs with different exposed facets might give BN materials special activities, facilitating their potential usage and expanding their range of applications. Though it is generally known that during the formation processes of nanocrystals, the crystal planes or edges with high surface energies grow faster, but ultimately these do not remain. Accordingly, the synthesis of BN NCs with other unusual exposed facets has become an important and challenging task. Up to now, although hollow polyhedron19 and polyhedral boron nitride nanofibers20 have been reported, BN materials with a uniform shapes and other indexed exposed planes have been little explored.
Different from CVD and similar techniques, the “autoclave route” was carried out in a sealed chamber. The special chemical environment (i.e. Na solvent,21a ZnCl2 active agent21b) as well as the successful synthesis examples21c of a series of BN nanomaterials enabled the “autoclave route” to be an efficient candidate for the synthesis of BN NCs with uniform shape and special exposed facets. Herein, we reported the synthesis of h-BN NCs with exposed {102} and {002} planes by an “autoclave route”. Considering the exposed {102} plane, high thermal stability and conductivity in nature, the gas phase selective oxidation of benzyl alcohol to benzaldehyde was evaluated here, and CL properties of the samples were also investigated, and the results indicated that it could be potentially used as an ultraviolent emitter material.
2 Experimental
2.1 Materials
All the reagents used in the experiments were purchased from Shanghai Chemical Reagents Co. NaN3 and FeCl3 were of CP grade, other reagents used were of AR grade.2.2 Sample preparation
In a typical procedure, 30 mmol NaBH4, 6.2 mmol FeCl3 and 40 mmol NaN3 were mixed and placed in a stainless steel autoclave of 20 mL capacity. The sealed autoclaves were heated in an electric oven from room temperature with an increasing rate of 10 °C min−1 to 750 °C and then maintained at 750 °C for 12 h. After that, it was cooled to room temperature naturally. The as-obtained powders were treated with distilled water and hydrochloric acid overnight. Then the as-obtained products were filtered and dried at 80 °C (sample S1).Caution: Since the reaction in the steel autoclave was carried out at 750 °C, to avoid the potential danger, the furnace and its controller were used in separated laboratories.
2.3 Sample characterization
X-ray powder diffraction (XRD) measurements were determined by a Bruker D8 advanced X-ray diffractometer. The morphology and structure of the products was investigated by field emission scanning electron microscopy (FE-SEM, FEI, Sirion200), transmission electron microscopy (TEM, Hitachi, H-7650) and high-resolution TEM (HRTEM, JEOL 2100). A CL spectrophotometer attached to the Sirion200 was used to investigate the optical properties of the synthesized BN nanomaterials. TG analysis was carried out using a TA SDT Q600 simultaneous thermogravimetric analyzer in the ambient atmosphere.2.4 Catalytic activity test
The catalytic performance of the product was measured by a continuous flow fixed-bed microreactor (vertical tubular reactor, i.d. = 4 mm) at atmospheric pressure. In all of the experiments, 40 mg catalysts were mixed with 25–50 mesh quartz sand (2 g). The simulated air stream (44 mL min−1, which was composed of 21% O2 and 79% N2) was supplied by mass flow controllers and benzyl alcohol (40 μL min−1) was fed continuously through a syringe pump. Liquid vaporization occurred in the preheater prior to the catalytic reaction bed. The outlet products and unreacted benzyl alcohol were cooled down using a cold trap (0 °C), collected, then analyzed by GC with a Shimadzu Type GC-14C equipped with a flame ionization detector, using a SGE-30QC2/AC5 capillary column and N2 as a carrier gas.3 Results and discussion
The phase purity and crystallinity of the as-prepared product were determined by XRD. The diffraction peaks in Fig. 1a with d-spacings of 3.33, 2.18, 2.07, 1.82, 1.67, 1.33 and 1.25 Å can be indexed as the diffraction crystal-planes of (002), (100), (101), (102), (004), and (103) from h-BN, with the calculated lattice constants a = 2.50 Å, c = 6.66 Å. No characteristic peaks associated with other crystalline byproducts were detected. It is well known that h-BN is a layered material, within each layer of h-BN, boron and nitrogen atoms are bound by strong covalent bonds, whereas the layers are held together by weak van der Waals’ forces. To characterize the degree of three-dimensional order of the h-BN, the Thomas definition for the graphitizing index was used:22| G.I. = [area (100) + area (101)]/area (102) | (1) |
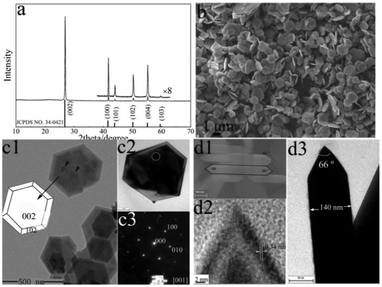 | ||
| Fig. 1 A typical XRD pattern of S1 (a). Typical SEM (b, d1) and TEM (c1, c2, d2) images of h-BN NCs. (b) Overview, (c1, c2) top view, Side view (d1, d2). The SEAD pattern in c3 corresponds to the circled area in c2, the HRTEM image of the tips in d2 is shown in d3. The bottom panel in (a) gives a JCPDS card of h-BN (No. 34-0421). | ||
Fig. 1b shows a typical large-area scanning electron microscope (SEM) image of the sample, indicating that the relatively homogeneous, well-shaped NCs were highly dominant (>90%). As shown in SEM and TEM images (Fig. 1b, c1, c2, d1, d3 and Fig. SI 2†), the as-prepared particles are uniform plates with polyhedral characteristics, which were composed of two hexagons and twelve side trapezoids. The mean side length of the NCs is 650 nm and the average thickness is ∼130 nm. As is shown in scheme in Fig. 1 c1, the top two surfaces could be indexed to (002) and (00−2). The side views of the BN NCs clearly show two tips, the measured intersection angle is 66 ± 4°, which matched well with that of the (012) and (01−2) planes. The SEAD patterns along the [001] (Fig. 2 c3) axis give a hexagonal symmetry spot pattern, indicating the single crystal nature of the BN NCs. The lattice spacing of 0.34 nm of the h-BN NCs in Fig. 1 d2 matches well with that of 0.33 nm for h-BN (002) planes.
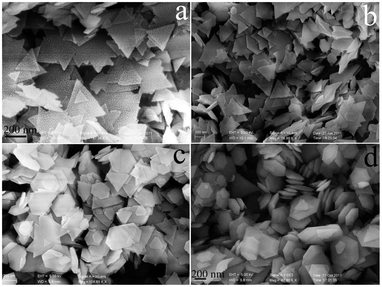 | ||
| Fig. 2 SEM images of the BN samples obtained at 720 °C with different reaction times: 30 min (a), 2 h (b), 4 h (c) and 12 h (d). | ||
According to the principle of crystal growth, planes with high surface energy and atomic density grow faster than the other facets, which are prone to ultimately disappearing. Actually, a higher reaction temperature results in the disappearance of the {012} plane (800 °C, 10 h. Fig. SI 3a†). Interestingly, the size of the hexagonal plates could be tuned by adjusting the reaction temperature in the range 710–760 °C, and the lower the reaction temperature, the smaller the NCs that could be obtained. For example, the mean side length of the hexagonal NCs was reduced to 250 nm at 730 °C for 10 h (Fig. SI 3b†).
The previous report of DFT calculation results suggests that “the equilibrium nanoparticles should be triangular with zigzag edges”.23 Our previous reports also prove that the hexagonal plates were originated from the N–H terminated regular nanoplates in a N2 atmosphere.9a In this study, the triagonal outline can be observed on the (002) plane of the BN NCs, as shown by an arrow in Fig. 1 c1. It gives us a clue that the BN NCs should originate from triagonal plates, and triagonal nanoplates were the dominant product in proper reaction conditions. To confirm the assumption, several control experiments were carried out at 720 °C. It is found that a shorter reaction time favours the formation of triagonal boron nitride nanoplates, Fig. 2a shows the SEM image of the BN obtained at 720 °C for 30 min, the triagonal nanoplates (>90%) with a mean side length of ∼300 nm were obtained in the majority. Besides the size increase of the triagonal plates (mean side length of 380 nm), the hexagon-like plates were obtained in a higher yield with a longer reaction time (2 h). The hexagonal like plates were the dominant product in the case of 4 h (Fig. 2c), and finally, the high yielding synthesis of the BN NCs can be realized after 12 h (Fig. 2d). It should be noted that the thickness of the starting plates was around 10–20 nm, it is nearly one tenth compared with that of BN NCs.
The CL luminescence emission of S1 was collected at room temperature to investigate its optoelectronic properties. The strong CL luminescence emission (Fig. 3a) in the ultraviolet range (center at 323 nm, 3.84 eV) can be attributed to the deep-level emissions associated with defects12b,24 The luminescence band around 585 nm might be related to the Fe doping (similar to Si or Eu doped BNNTs)25 caused by Fe-containing species used in the synthesis process. Fig. 3b depicts the SEM images of S1, the corresponding CL luminescence images recorded at 323 and 585 nm are given in Fig. 3c and Fig. 3d (d), respectively, indicating uniform optical properties across the whole sample. The strong ultraviolet optoelectronic properties of the BN NCs can be potentially applied as optoelectronics in the ultraviolet region.
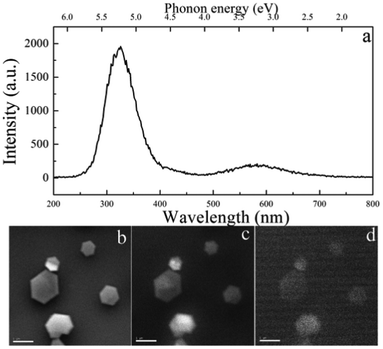 | ||
| Fig. 3 (a) The CL spectrum of S1 measured at room temperature. A SEM image (b) and corresponding CL images of S1 recorded at 323 nm (c) and 585 nm (d). | ||
For many uses and applications, the stability of the BN material is very important. To investigate the thermal stability of the as-obtained S1, the TGA analysis was carried out in an ambient atmosphere (Fig. 4a). It was found that S1 is rather stable below 1000 °C, no obvious weigh gain or loss is observed. The weight gain at 1000 °C can be ascribed to the oxidation of BN. The TGA curve of the boron nitride nanosheets is also given in Fig. 4a for comparation,21b which shows obvious weight loss and gain. Furthermore, S1 is stable around 1200 °C in flowing N2, and the morphology of the BN NCs is truncated and is transformed to round-like plates around 1500 °C for 2 h.
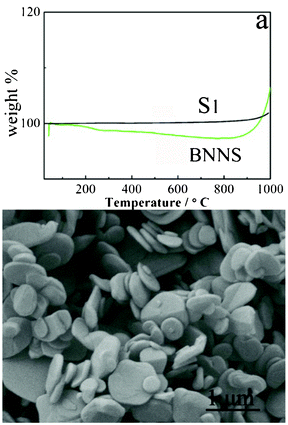 | ||
| Fig. 4 TGA curves of the BN NCs (black) and nanosheets (green)21b (a), and the typical SEM image of the sample calcined at 1500 °C for 2 h in flowing N2. | ||
Selective oxidation of alcohols to aldehydes is a crucial process for the synthesis of fine chemicals. The gas phase selective oxidation of benzyl alcohol using a supported and unsupported gold catalyst by eco-friendly air results in relatively satisfactory conversion and selectivity.26–28 On account of the high conductivity, thermal stability, and exposed (012) planes, it is reasonable to consider that the Au/BNNCs composite would be effective for the selective oxidation of benzyl alcohol to benzaldehyde.
Fig. 5a top inset shows an overview TEM image of the Au/BN NCs, an enlarged TEM image in Fig. 5a indicates that the supported Au nanoparticles with diameters ranging from 1–8 nm (see Fig. 5a bottom inset, mean diameter of 2.5 nm). Fig. 5b shows the activity profiles of 5 wt% Au/BN NCs and BN NCs towards the selective oxidation of benzyl alcohol to benzaldehyde by air (44 mL min−1) at 240 °C. Though only 40 mg of the Au/BN NCs were used here (containing ∼2 mg Au), the conversion and selectivity were ∼30%, ∼98%, respectively, which is a bit better than those of unsupported nanoporous pure gold at 240 °C (conversion 23%, selectivity 98%).27a In accordance with our very recent reports,27b it is found that an initial conversion of ∼15% and selectivity of 99% could also be obtained with 40 mg of the BN NCs sample (Fig. 5b). Whereas, control experiments indicated that only less than 1% products can be obtained when the same process was carried out using pure quartz sand. It is considered that the BN support plays a positive role in the relatively highly selective oxidation of benzyl alcohol to benzaldehyde; however, the exact catalytic mechanism still needs further research.
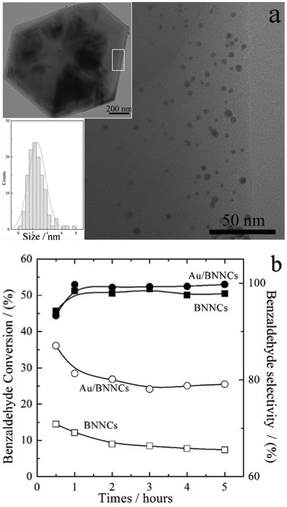 | ||
| Fig. 5 TEM images of 5%wt Au/BN samples, overview (a, top inset), enlarged view and particles sizes distribution of Au on the BNNCs (a, bottom inset). (b) The catalytic oxidation of benzyl alcohol to benzaldehyde by the 5%wt Au/BN samples and the black BNNCs, solid symbols for selectivity, hollow symbols for conversion. The tests were carried out at 240 °C. | ||
4 Conclusions
In this report, hexagonal BN NCs with exposed {102} planes were synthesized. The CL properties of the samples enable it to be potentially used in the areas of ultraviolet emitter materials. The gas phase selective oxidation of benzyl alcohol to benzaldehyde by Au/BN NCs was taken as an example, and a relatively high conversion (∼30%) and selectivity (98%) at 240 °C were detected.Acknowledgements
This work was supported by the National Nature Science Found of China (20971079), Academy of Sciences large apparatus United Fund (No. 11179043), and the 973 Project of China (No. 2011CB935901). Thanks Prof. Dr. Yao Xu and Chunli Guo for helpful discussion, Dr. Meng Wang and Lulu Si for proofing the manuscript.References
- (a) C. Zhi, N. Hanagata, Y. Bando and D. Golberg, Chem.–Asian J., 2011, 6(9), 2530–2535 CrossRef CAS; (b) C. Y. Zhi, Y. Bando, T. Terao, C. C. Tang, H. Kuwahara and D. Golberg, Chem.–Asian J., 2009, 4(10), 1536–1540 CrossRef CAS; (c) C. Zhi, Y. Bando, T. Terao, C. Tang, H. Kuwahara and D. Golberg, Adv. Funct. Mater., 2009, 19(12), 1857–1862 CrossRef CAS.
- Y. Kubota, K. Watanabe, O. Tsuda and T. Taniguchi, Science, 2007, 317, 932–934 CrossRef CAS.
- (a) C. Tang, Y. Bando, Y. Huang, C. Zhi and D. Golberg, Adv. Funct. Mater., 2008, 18, 3653–3661 CrossRef CAS; (b) V. Salles, S. Bernard, J. Li, A. Brioude, S. Chehaidi, S. Foucaud and P. Miele, Chem. Mater., 2009, 21, 2920–2929 CrossRef CAS.
- C. Y. Zhi, Y. Bando, C. C. Tang, Q. Huang and D. Golberg, J. Mater. Chem., 2008, 18, 3900 RSC.
- (a) Y. J. Qiu, J. Yu, J. Rafique, J. Yin, X. D. Bai and E. Wang, J. Phys. Chem. C, 2009, 113, 11228–11234 CrossRef CAS; (b) J. Wang, L. Zhang, G. Zhao, Y. Gu, Z. Zhang, F. Zhang and W. Wang, J. Solid State Chem., 2011, 184, 2478–2484 CrossRef CAS.
- V. Salles, S. Bernard, A. Brioude, D. Cornu and P. Miele, Nanoscale, 2010, 2, 215 RSC.
- G. M. Ingo, G. Padeletti, T. de Caro, C. Riccucci, F. Faraldi, A. Curulli, A. Mezzi and M. Piccinini, J. Mater. Chem., 2011, 21, 10268 RSC.
- J. Yu, L. Qin, Y. Hao, S. Kuang, X. Bai, Y.-M. Chong, W. Zhang and E. Wang, ACS Nano, 2010, 4, 414–422 CrossRef CAS.
- (a) M. Li, L. Xu, C. Sun, Z. Ju and Y. Qian, J. Mater. Chem., 2009, 19, 8086 RSC; (b) L. Q. Xu, J. H. Zhan, J. Q. Hu, Y. Bando, X. L. Yuan, T. Sekiguchi, M. Mitome and D. Golberg, Adv. Mater., 2007, 19, 2141–2144 CrossRef CAS; (c) Z. Zhao, Z. Yang, Y. Wen and Y. Wang, J. Am. Ceram. Soc., 2011, 94, 4496–4501 CrossRef CAS.
- L.-x. Lin, Y. Zheng, Y. Zheng and K.-m. Wei, Mater. Lett., 2007, 61, 1735–1737 CrossRef CAS.
- G. Lian, X. Zhang, M. Tan, S. Zhang, D. Cui and Q. Wang, J. Mater. Chem., 2011, 21, 9201 RSC.
- (a) M. Wang, M. Li, L. Xu, L. Wang, Z. Ju, G. Li and Y. Qian, Catal. Sci. Technol., 2011, 1, 1159–1165 RSC; (b) B. Zhong, Y. Wu, X. Huang, G. Wen, H. Yu and T. Zhang, CrystEngComm, 2011, 13, 819 RSC; (c) C. Zhi, Y. Bando, C. Tang, D. Golberg, R. Xie and T. Sekiguchi, Appl. Phys. Lett., 2005, 87(6), 063107 CrossRef.
- C. Jacobsen, J. Catal., 2001, 200, 1–3 CrossRef CAS.
- K. Zhou and Y. Li, Angew. Chem., Int. Ed., 2012, 51, 602–613 CrossRef CAS.
- X. Xie, Y. Li, Z.-Q. Liu, M. Haruta and W. Shen, Nature, 2009, 458, 746–749 CrossRef CAS.
- J. Yin, Z. Yu, F. Gao, J. Wang, H. Pang and Q. Lu, Angew. Chem., Int. Ed., 2010, 49, 6328–6332 CrossRef CAS.
- B. L. Lv, Z. Y. Liu, H. Tian, Y. Xu, D. Wu and Y. H. Sun, Adv. Funct. Mater., 2010, 20, 3987–3996 CrossRef CAS.
- (a) N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS; (b) Y. Ma, Q. Kuang, Z. Jiang, Z. Xie, R. Huang and L. Zheng, Angew. Chem., 2008, 120, 9033–9036 CrossRef.
- S. Bernard, V. Salles, J. Li, A. Brioude, M. Bechelany, U. B. Demirci and P. Miele, J. Mater. Chem., 2011, 21, 8694 RSC.
- L.-x. Lin, Y. Zheng, Z.-h. Li, X.-n. shen and K.-m. Wei, Solid State Sci., 2007, 9, 1099–1104 CrossRef CAS.
- (a) L. C. Wang, L. Q. Xu, C. H. Sun and Y. T. Qian, J. Mater. Chem., 2009, 19, 1989–1994 RSC; (b) L. Wang, C. Sun, L. Xu and Y. Qian, Catal. Sci. Technol., 2011, 1, 1119–1123 RSC; (c) L. Xu, S. Li, Y. Zhang and Y. Zhai, Nanoscale, 2012, 4, 4900–4915 RSC.
- (a) J. Thomas, N. E. Weston and T. E. O'Connor, J. Am. Chem. Soc., 1962, 84, 4619–4622 CrossRef CAS; (b) M. G. Balint and M. I. Petrescu, Diamond Relat. Mater., 2009, 18, 1157–1162 CrossRef CAS.
- Y. Liu, S. Bhowmick and B. I. Yakobson, Nano Lett., 2011, 11, 3113–3116 CrossRef CAS.
- (a) M. Silly, P. Jaffrennou, J. Barjon, J. S. Lauret, F. Ducastelle, A. Loiseau, E. Obraztsova, B. Attal-Tretout and E. Rosencher, Phys. Rev. B, 2007, 75 Search PubMed; (b) C. Zhi, Y. Bando, C. Tang, D. Golberg, R. Xie and T. Sekigushi, Appl. Phys. Lett., 2005, 86, 213110 CrossRef.
- B. Zhong, X. Huang, G. Wen, L. Xia, H. Yu and H. Bai, J. Phys. Chem. C, 2010, 114, 21165–21172 CAS.
- C. Zhi, Y. Bando, G. Shen, C. Tang and D. Golberg, J. Nanosci. Nanotechnol., 2007, 7, 530–534 CrossRef CAS.
- (a) D. Q. Han, T. T. Xu, J. X. Su, X. H. Xu and Y. Ding, ChemCatChem, 2010, 2, 383–386 CrossRef CAS; (b) Y. X. Zhang, L. Q. Xu, B. Tang and Z. W. Li, Catal. Sci. Technol., 2012 10.1039/C2CY20122D.
- J. Zhu, S. A. C. Carabineiro, D. Shan, J. L. Faria, Y. Zhu and J. L. Figueiredo, J. Catal., 2010, 274, 207–214 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: XRD pattern in the range 10–90°, FT-IR and Raman spectra, SEM images. See DOI: 10.1039/c2ra21325g |
| This journal is © The Royal Society of Chemistry 2012 |
