Underwater bonding strength of marine mussel-inspired polymers containing DOPA-like units with amino groups†
Feng
Zhang
a,
Siwei
Liu
*a,
Yi
Zhang
a,
Yen
Wei
*b and
Jiarui
Xu
a
aPCFM Lab, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, 510275, China. E-mail: liusiw@mail.sysu.edu.cn
bDepartment of Chemistry, Tsinghua University, Beijing, 100084, China. E-mail: weiyen@tsinghua.edu.cn
First published on 8th August 2012
Abstract
It is widely accepted that the bonding strength of mussel protein is somehow related to the contained pyrocatechol groups. However, by comparing the bonding strengths of synthetic terpolymers before and after neutralization by a diamine, we found that the amino groups may have a great influence on the bonding strength, in addition to the pyrocatechol groups.
The glue proteins produced by marine mussels can bind strongly to almost all organic and inorganic surfaces, including, in almost all cases, both the wet and dry environments. The studies of these functionally unique proteins have revealed that the presence of the unusual amino acid 3,4-dihydroxy-L-phenylalanine (DOPA) is vital to their adhesive properties.1 The content of DOPA has an essential impact on the adhesive properties because DOPA is central to the cross-linking reaction of curing and surface bonding.1,2 It is generally believed that DOPA adhesion is somehow related to the presence of pyrocatechol, which possesses adjacent hydroxyl groups on the benzene ring. However, the detailed bonding mechanisms of DOPA on various surfaces remain unknown.
By mimicking the glue proteins generated by marine mussels, a number of synthetic polymers containing DOPA (or DOPA-like) units have been synthesized successfully. This could lead to the widespread use of mussel glue protein-like polymers as surgical adhesives. However, as the binding mechanism remains unknown, the design and development of such “bio-glue” materials from the molecular level to practical application lacks clear direction, especially in terms of bonding strength and speed.
In the mussel adhesive proteins, lysine may provide reactivity, with oxidized DOPA, for the cross-linking reaction in the adhesives. However, previous reports by Suci and Burzio et al. have shown that lysine did not appear to participate in the multimer formation for the DOPA-containing proteins and peptides.3 Wilker believed that the role of the lysine residues in the mussel adhesive protein was probably only to provide the cationic charges.4 Although the terpolymers containing ammonium groups synthesized by the Wilker group had appreciable adhesion (shear strength), the underwater adhesion strength was rather weak, about 10% of that measured under dry conditions.
Up to now, almost all the reported adhesive polymers containing DOPA-like units have used amines as the functional groups (with most of them being secondary amines), except for a very few without any amino groups.5 Pelton5a reported that the synthetic polymer poly[(sodium 4-styrenesulfonate)-co-(2-methoxy-4-vinylphenol)], could participate in a crosslinking reaction through the methoxy group substituted styrene, resulting in the production of an amount of gel (crosslinks) during the copolymerization. However, the crosslink structures were not known. In 2007, Wilker5b presented a simplified polymer mimic of cross-linking adhesive proteins, poly(3,4-dihydroxystyrene-co-styrene). Without a polypeptide backbone or a variety of amino acid side groups, this polymer appeared to crosslink in a manner analogous to the mussel adhesive proteins. The shear adhesive strength (under dry conditions) of this polymer was ca. 0.6 MPa, and could be increased upto 1.2 MPa after oxidition with Cr2O72−. A styrene–butadiene random copolymer terminated with 3,4-dihydroxybenzaldehyde (DOBA) was prepared by the Pan group.5c They found that the DOBA end-group exhibited a strong affinity toward various fillers, such as carbon black and hydrophilic inorganic oxides, and could be exploited for beneficial modification of polymer–filler interactions. To construct adhesive polymers deriving from marine mussel proteins, DOPA-like units containing amino-groups have usually been selected. This may be due to two reasons: (i) amino is an active group which can be easily incorporated into the polymer chain, and (ii) from the bionic perspective this unit is very similar to the DOPA moiety in the marine mussel proteins.
Analysis of the possible reactions in the adhesive processes has led us to speculate that the adhesion of mussel proteins may also be related to the presence of amino groups in addition to pyrocatechol. Usually, these amino groups in the mussel proteins exist in form of secondary amines (–NH–). The polymers containing such DOPA-like units (such as amino groups) could be readily positively charged. However, the charged amino group and the amino group with a lone pair of electrons are reversible, and thus the “Michael addition” reaction could take place (Scheme 1). Thus, it is not unreasonable to assert that the adhesion of these polymers is mainly determined by the positively charged amino groups or the cross-linking reaction through Michael addition.
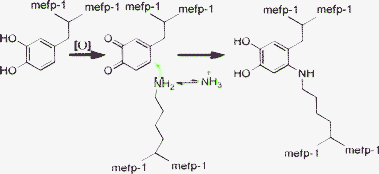 | ||
| Scheme 1 Oxidation of catechol (DOPA-like units) is followed by nucleophilic addition of amino groups of lysine residues. | ||
To validate our speculation, four terpolymers with similar structures were synthesized via free radical polymerization using azodiisobutyronitrile (AIBN) as a catalyst (Fig. 1 and Table 1). The building blocks of the terpolymers are N-(3,4-dihydroxyphenethyl) methacrylamide (DMA),2h acrylic acid (AA) and butyl acrylate (BA). The molar ratio of DMA ranges from 19.24 to 48.39. The maximum tensile strength of these copolymers is ca. 3.8 MPa in the dry environment. Interestingly, in the wet environment, the maximum adhesive strength could be increased almost two times (from 0.8 to 1.4 MPa) (Tables 1 and 2) when these terpolymers are neutralized by excess diamine (compared to the amount of AA). The free radical polymerizations were carried out in dimethylformamide at 70 °C. The results are summarized in Table 1. The data of the molecular weights were measured by using the non-neutralized terpolymers. In the 1H NMR spectra, some peaks in the range of 6.5∼6.7 ppm appear, corresponding to the characteristic peaks of DMA.6 By comparing the integral area of DMA (aromatic proton), methylene that directly linked to the carbonyl group in the backbone, and the methylene tethered to the ester group in BA, we may evaluate the DMA contents in these terpolymers. The molar ratios of DMA in the terpolymers calculated by NMR measurements are somewhat smaller than those calculated by the feed ratios, i.e., 18.07, 27.48, 38.18, and 45.73 for Runs 1–4, respectively (as compared to those in Table 1). This is probably due to the different reactivity of each vinyl monomer.
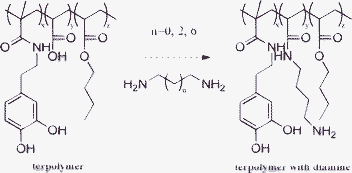 | ||
| Fig. 1 The synthesized terpolymers. Because of the carboxyl in the acrylic acid, the terpolymers could be neutralized by diamines easily. The diamines used here are 1,2-ethanediamine (ED), 1,4-butanediamine (BD) and 1,8-octanediamine (OD), respectively. | ||
| Run | Volume of use (g) | M n (×104) | DPI | Molar ratio of DMAa | Molar ratio of AA | T g (°C) | Maximum adhesion(MPa)b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMA | AA | BA | AIBN | Dryc | Wetc | Dryd | Wetd | ||||||
| a The molar ratio of DMA was calculated by the feed ratio of each experiment. b The maximum adhesion was determined on an Instron under various conditions, such as dry adhesion and underwater adhesion. c The copolymers themselves. d The copolymers neutralized by 1,4-butanediamine. | |||||||||||||
| 1 | 0.4260 | 0.4163 | 0.2954 | 0.0100 | 7.9 | 1.4 | 19.24 | 57.7 | 6.7 | 1.9 ± 0.2 | 0.3 ± 0.1 | 1.8 ± 0.3 | 0.5 ± 0.2 |
| 2 | 0.4685 | 0.2507 | 0.1832 | 0.0100 | 8.2 | 1.5 | 30.14 | 49.5 | 3.6 | 2.3 ± 0.2 | 0.7 ± 0.3 | 2.4 ± 0.2 | 1.0 ± 0.2 |
| 3 | 0.4440 | 0.1612 | 0.1211 | 0.0100 | 8.6 | 1.6 | 38.68 | 42.7 | 4.4 | 3.8 ± 0.5 | 0.8 ± 0.3 | 3.9 ± 0.6 | 1.4 ± 0.3 |
| 4 | 0.4365 | 0.1060 | 0.0812 | 0.0100 | 9.2 | 1.4 | 48.39 | 36.8 | −6.8 | 2.8 ± 0.4 | 0.12 ± 0.2 | 3.0 ± 0.3 | 0.2 ± 0.2 |
| Run | T g (°C) | M n (×104) | DPI | Maximum adhesion (MPa)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED | BD | OD | ED | BD | OD | ED | BD | OD | ED | OD | |||
| Dry | Wet | Dry | Wet | ||||||||||
| a The maximum adhesion was determined on an Instron under different circumstances of the polymers neutralized by a diamine. | |||||||||||||
| 1 | 10.6 | 14.7 | 11.2 | 8.1 | 8.2 | 8.5 | 1.4 | 1.4 | 1.5 | 2.0 ± 0.2 | 0.7 ± 0.2 | 1.9 ± 0.2 | 0.6 ± 0.1 |
| 2 | 9.0 | 8.9 | 8.7 | 8.3 | 8.3 | 8.3 | 1.6 | 1.5 | 1.6 | 2.3 ± 0.3 | 1.0 ± 0.2 | 2.6 ± 0.2 | 1.3 ± 0.3 |
| 3 | 8.6 | 11.7 | 7.3 | 8.6 | 8.7 | 8.7 | 1.6 | 1.6 | 1.5 | 4.0 ± 0.7 | 1.3 ± 0.5 | 3.9 ± 0.5 | 1.2 ± 0.3 |
| 4 | 1.5 | 2.6 | 4.3 | 9.2 | 9.3 | 9.3 | 1.4 | 1.4 | 1.5 | 2.8 ± 0.4 | 0.15 ± 0.4 | 2.7 ± 0.4 | 0.16 ± 0.2 |
The glass transition temperature (Tg) was measured by differential scanning calorimetry. The Tg values of these copolymers are all lower than 10 °C. A lower Tg is of great benefit to the wettability and liquidity of these terpolymers. All the terpolymers have good liquidity at room temperature; therefore, the polymers are easily prone to fill the gaps between the bonding interfaces, probably resulting in bonding strength improvement.7 As for the terpolymer in Run 3, the Tg of the non-neutralized sample is 4.4 °C. If it is neutralized by 1,4-butanediamine, the Tg is increased from 4.4 to 11.7 °C. The other three terpolymers neutralized by the diamine have the similar trends in the Tg variation; however, the range of variation is usually less than 10 °C.
Among the prior studies on mussel adhesive mimics, most are concerned with the micro-bonding strength, with a few interested in the bulk bonding,8 and even fewer works concerned with mussel adhesive mimics in a wet environment.4,8d The influences of amino group on the bonding strength of such mussel adhesives have not yet been investigated by far.
The bonding strength (in the form of tensile strength testing) of the synthesized terpolymers was recorded on an Instron (model CMT6103 of Sunthink Co. Ltd.) machine. For each sample, the tensile strength data were averaged of at least five measurements, and the results are listed in Tables 1 and 2. The bonding strength increases along with the increase of DMA content in the synthesized terpolymers. When the molar ratio of DMA is about 40%, the strength reaches a maximum of 3.8 MPa (dry adhesion, without neutralization by the diamine). However, the bonding strength gradually declines with further increasing of the content of DMA. When the tensile strength tests were carried out in the wet environment, the bonding strength had the same variation tendency, with the maximum bonding strength (0.8 MPa) found at a DMA molar ratio of around 38%.
When the synthesized terpolymers were neutralized by the diamine, the dry adhesive strength did not undergo any obvious changes. However, the wet adhesion was substantially increased. As for Run 3 terpolymer, the wet adhesion increases from 0.8 to 1.3 MPa (neutralized by BD, +62%). By using different diamines, the bonding strength can also be improved in similar trends; but it seems to be irrelevant to the type of diamine (in our experiments we chose diamines of different molecular sizes to neutralize the terpolymers). We thus speculate that the function of the diamine might be to provide amino groups, and the amino groups may have a great influence on the adhesion, especially in a wet environment. In other words, it is likely that the bonding strength has no direct relationship to the specific diamine, except for the amino groups tethered to the polymer.
These non-neutralized terpolymers contain the secondary amine and catechin (catecholamine) groups. Many previous studies on such polymers mainly focused on the adhesive function of the pyrocatechol groups, with no concern about the effect of the amino groups on adhesion (dry or wet; bulk or micro). By comparing the adhesion of the terpolymers with the terpolymers neutralized by the diamine, we could infer that the amino groups may have a great influence on the bulk bonding, especially in the wet environment. The amino groups can act as nucleophilic reagents to attack the oxidative catechol, forming crosslinked products, as illustrated in Scheme 1. On the other hand, for the terpolymers that are not neutralized by the diamine, only one secondary amine group exists in each of the DMA segments, and the amino group can form a five-membered ring as illustrated in Scheme 2.9 This process mainly occurs in the compounds with one DOPA-like unit (intramolecular), whereas the chains of terpolymers neutralized by the diamine contain many primary amine groups besides the secondary amine. The primary amine may attack the oxidative DOPA-like unit as a nucleophilic reagent through an intra- or intermolecular reaction, resulting in a crosslinked product. Therefore, a stronger adhesion may be achieved compared to the non-neutralized terpolymers. From these experimental results, it is our belief that the bonding strength of these terpolymers is indirectly related to the content of amino groups (which can be estimated from the molar ratio of AA) tethered to the polymers. Additionally, the adhesion in the dry environment of the copolymers exhibits no obvious changes when neutralized by the diamine. We are not able to give a convincing explanation for this at present without further investigation.
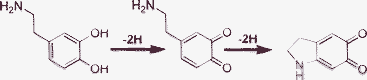 | ||
| Scheme 2 Formation of a quinone from dopamine. Dopamine could form a five ring intermediate through the reaction between the amino group and benzene ring (intramolecular).9 | ||
In summary, a new approach has been developed and used to prepare terpolymers containing DMA and amino groups (primary amines) with different contents. The bulk dry adhesion increases with a corresponding increase in DMA content, and reaches a maximum at around 40 mol%. The bonding strength in the wet environment can be remarkablely increased when the terpolymers are neutralized by a diamine. This finding is valuable for further exploitation of applications such as novel bio-adhesives and underwater bonding. This approach also provides a useful research methodology for investigation of the adhesive mechanism of mussel foot proteins and molecular designs of new adhesive materials used in wet environments.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 50903096 to SWL, and No. 21134004 to YW), the Department of Science Technology of Guangdong Province (2008B090500196), and the Fundamental Research Funds for the Central Universities.References
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999 CrossRef CAS.
- (a) Q. Lin, D. Gourdon, C. J. Sun, N. Holten-Andersen and T. H. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3782 CrossRef CAS; (b) O. A. Akemi and R. L. Garrell, BioPolymers, 2000, 57, 91 Search PubMed; (c) T. H. Anderson, J. Yu, A. Y. Estrada, M. U. Hammer, J. H. Waite and J. N. Israelachvili, Adv. Funct. Mater., 2010, 20, 4196 CrossRef CAS; (d) J. Yu, W. Wei, E. Danner, R. M. Ashley, J. Israelachvili and J. H. Waite, Nat. Chem. Biol., 2011, 7, 588 CrossRef CAS; (e) D. S. Hwang, H. Zeng, A. Masic, M. J. Harrington, J. N. Israelachvili and J. H. Waite, J. Biol. Chem., 2010, 285, 25850 CrossRef CAS; (f) J. H. Waite and X. X. Qin, Biochemistry, 2001, 40, 2887 CrossRef CAS; (g) H. Zhao and J. H. Waite, Biochemistry, 2006, 45, 14223 CrossRef CAS; (h) H. Lee, B. P. Lee and P. B. Messersmith, Nature, 2007, 448, 338 CrossRef CAS.
- (a) P. A. Suci and G. G. Geesey, J. Colloid Interface Sci., 2000, 230, 340 CrossRef CAS; (b) L. A. Burzio and J. H. Waite, Protein Sci., 2001, 10, 735 CrossRef CAS.
- J. D. White and J. J. Wilker, Macromolecules, 2011, 44, 5085 CrossRef CAS.
- (a) Y. Zhuo and P. Robert, Macromol. Rapid Commun., 1998, 19, 241 CrossRef; (b) G. Westwood, T. N. Horton and J. J. Wilker, Macromolecules, 2007, 40, 3960 CrossRef CAS; (c) X. D. Pan, Z. Qin, Y. Y. Yan and P. Sadhukhan, Polymer, 2010, 51, 3453 CrossRef CAS.
- M. Guvendiren, P. B. Messersmith and K. R. Shull, Biomacromolecules, 2008, 9, 122 CrossRef CAS.
- F. Zhang, S. W. Liu, Y. Zhang, J. R. Xu and Z. G. Chi, Int. J. Adhes. Adhes., 2011, 31, 583 CrossRef CAS.
- (a) M. Yu and T. J. Deming, Macromolecules, 1998, 31, 4739 CrossRef CAS; (b) J. Wang, C. Liu, X. Lu and M. Yin, Biomaterials, 2007, 28, 3456 CrossRef CAS; (c) H. Shao, K. N. Bachus and R. J. Stewart, Macromol. Biosci., 2009, 9, 464 CrossRef CAS; (d) H. Shao and R. J. Stewart, Adv. Mater., 2010, 22, 729 CrossRef CAS; (e) S. A. Burke, M. Ritter-Jones, B. P. Lee and P. B. Messersmith, Biomed. Mater., 2007, 2, 203 CrossRef CAS; (f) J. L. Murphy, L. Vollenweider, F. Xu and B. P. Lee, Biomacromolecules, 2010, 11, 2976 CrossRef CAS.
- (a) L. C. Jean and B. Christie, Neurochem. Int., 1998, 32, 117 Search PubMed; (b) H. Zhao, N. B. Robertson, S. A. Jewhurst and J. H. Waite, J. Biol. Chem., 2006, 281, 11090 CrossRef CAS; (c) Y. Jing, W. Wei, E. Danner, R. K. Ashley, J. N. Israelachvili and J. H. Waite, Nat. Chem. Biol., 2011, 7, 588 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: the synthesis of the terpolymers and sample preparation for the tensile strength test are included. See DOI: 10.1039/c2ra21312e/ |
| This journal is © The Royal Society of Chemistry 2012 |
