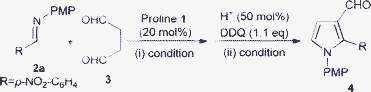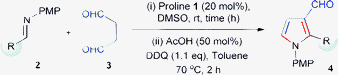Organocatalytic Mannich/cyclization/aromatization sequence: direct synthesis of substituted pyrrole-3-carboxaldehydes†‡
Indresh
Kumar
*ab,
Nisar A.
Mir
a,
Panduga
Ramaraju
a and
Basant P.
Wakhloo
c
aDepartment of Chemistry, Birla Institute of Technology & Science, Pilani 333 031, Rajasthan, India. E-mail: indresh.chemistry@gmail.com; indresh.kumar@bits-pilani.ac.in; Fax: +91 1596 244183; Tel: +91 1596 245073 Ext. 276
bSchool of Biology & Chemistry, College of Science, Shri Mata Vaishno Devi University, Katra 182 320, India
cInstrumentional Division, IIIM-CSIR Lab, Jammu 180 001, India
First published on 8th August 2012
Abstract
A robust method for the synthesis of substituted pyrrole-3-carboxaldehydes from N-PMP aldimines and succinaldehyde is reported. This reaction involves an organocatalytic direct Mannich reaction, and an acid catalyzed cyclization and oxidative aromatization sequence with high yields (up to 82%).
Pyrroles1 are well-known heterocycles that are present in numerous biologically active natural as well as synthetic compounds.2 These show many biological activities, such as antibacterial,3a antiviral,3b antitumor,3c anti-oxidative,3d anti-inflammatory3e activity as well as multiple applications in materials science.4 The Paal–Knorr condensation is one of the classical methods for the synthesis of pyrroles, where the 1,4-dicarbonyl unit provides four carbon atoms of the ring and an amine group provides the nitrogen atom with the N-substituent (eqn (1), Fig. 1).5
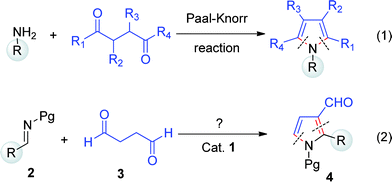 | ||
| Fig. 1 Direct approaches to substituted pyrroles from 1,4-dicarbonyls. | ||
However, the problems and limitations, such as the harsh cyclization conditions,5g associated with the Paal–Knorr method have driven the development of other methods, which mainly rely on cycloaddition,6 multi-component,7 and metal-mediated reactions.8 Although there are a few indirect routes,9a–c none of the methods offer direct access to substituted pyrrole-3-carboxaldehyde,9d even though these compounds are important synthetic intermediates.10a–c Direct substitution at the C-3 position of pyrrole,10d,e particularly, direct formylation at C-3, remains a challenge because electrophilic substitutions mainly occur at the C-2 position. Therefore, a general strategy to synthesize pyrrole-3-carboxaldehydes from simple building blocks with a minimal number of overall synthetic steps is highly desirable. Additionally, it is also motivating to develop a new method with variation, where 1,4-dicarbonyl compounds serve as the source of the three ring atoms and the other two atoms of the pyrrole moiety could be obtained from imines (eqn (2), Fig. 1).
Organocatalytic cascade reactions involving two or more selective transformations using single/multiple catalysis, serve as powerful tools to conserve energy and minimize the number of synthetic operations.11 With the idea of cascade synthesis for pyrroles in mind, we reasoned that a simple catalytic route for substituted pyrrole-3-carboxaldehydes could be developed from succinaldehyde and imines. Although, imines have been used earlier to synthesize pyrroles,12 to the best of our knowledge, this is the first report to employ such building blocks: aldimines and succinaldehyde. Herein, we report an entirely new synthetic method for the synthesis of substituted pyrrole-3-carboxyaldehdyes, which consists of the following steps viz. a direct Mannich reaction, followed by acid catalyzed cyclization and oxidative aromatization, as a two-step sequence.
As part of our recent interest in the development of synthetic methods for heterocyclic compounds,13 we anticipated that an aqueous solution of succinaldehyde 3,14 a synthetically useful 1,4-dicarbonyl unit, might participate in a cascade sequence similar to aqueous glutaraldehyde used by Hayashi and co-workers for other reactions.15 The optimization study for this direct approach to synthesize pyrrole-3-carboxaldehdye were conducted using N-PMP aldimine 2a pre-formed from p-nitrobenzaldehyde as shown in Table 1.
|
|
||||
|---|---|---|---|---|
| Entry | 3 (x M sol.)b | Steps | Conditionsa | Yield (%)c |
| a (i) Imine 2 (0.3 mmol), 3 (× M sol, 0.9 mmol), 1 (20 mol%), solvent (3.0 mL); (ii) CH3CO2H (50 mol%, 0.15 mmol), solvent (3.0 mL) DDQ (1.1 eq). b See the ESI.† c Isolated yields refer to 2 (after two steps). d 1 (10 mol%). e CH3CO2H (not added). f Pyrrolidine (20 mol%), CH3CO2H (20 mol%) were used in place of 1. | ||||
| 1 | 6 M | i | DMF, rt, 10 h | |
| ii | DMF, rt, 24 h | <20 | ||
| 2 | 6 M | i | DMF, rt, 10 h | |
| ii | benzene, 40 -C, 6 h | 51 | ||
| 3 | 6 M | i | CH3CN, rt, 12h | |
| ii | CH3CN, 40 -C, 6 h | 34 | ||
| 4 | 6 M | i | DMSO, rt, 9 h | |
| ii | DMSO, 40 -C, 6 h | <20 | ||
| 5 | 6 M | i | DMSO, rt, 9 h | |
| ii | benzene, 40 -C, 6 h | 54 | ||
| 6 | 6 M | i | DMSO, rt, 9 h | |
| ii | Toluene, 60 -C, 4 h | 62 | ||
| 7 | 4 M | i | DMSO, rt, 7 h | |
| ii | Toluene, 60 -C, 4 h | 73 | ||
| 8 | 3 M | i | DMSO, rt, 6 h | |
| ii | Toluene, 70 -C, 2 h | 82 | ||
| 9d | 3 M | i | DMSO, rt, 10 h | |
| ii | Toluene, 70 -C, 2 h | 63 | ||
| 10 | 2 M | i | DMSO, rt, 6 h | |
| ii | Toluene, 70 -C, 2 h | 65 | ||
| 11e | 3 M | i | DMSO, rt, 6 h | |
| ii | Toluene, 70 -C, 2 h | 49 | ||
| 12f | 3 M | i | DMSO, rt, 12 h | |
| ii | Toluene, 70 -C, 2 h | 43 | ||
During the initial experimental studies, we found that proline 1 catalyzed the direct Mannich reaction of aqueous succinaldehyde 3 with imine 2a, followed by acid catalyzed cyclization and DDQ mediated aromatization as a two-step sequence to afford substituted pyrrole 3-carboxaldehyde 4a in high yield (entry 8, Table 1). The amount of water present alters the course of the reaction (entry 6–8 and 10, Table 1), similar to that discussed earlier by Barbas and others for direct organocatalytic Mannich reactions.16 Additionally, we also examined other solvent combinations (entry 1–6, Table 1) and found that DMSO/toluene was optimal for this two-step process. Furthermore, decreasing the catalyst loading (entry 9, Table 1), and using a different catalytic system (entry 12, Table 1), led to prolonged reaction times with reduced yield due to the lability of the imine in the presence of water. The reaction proceeded with low yield in the absence of acid (entry 11, Table 1), which shows that the presence of acid is essential for enhancing the rate of the intramolecular cyclization. Thus, we preferred to perform this two-step sequence with the optimized conditions (entry 8, Table 1).
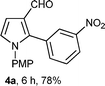
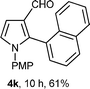
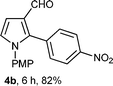
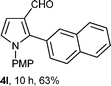
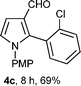
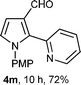
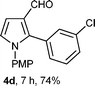
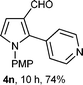
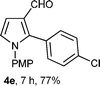
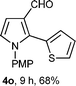
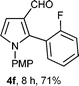
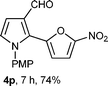
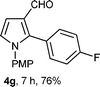
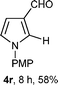
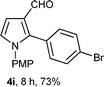
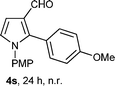
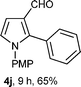
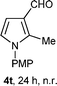
With the optimal conditions in hand, we next examined the generality of this developed transformation by employing various N-PMP aldimines and the results are summarized in Table 2. The reaction proceeded with high yields in case of electron-deficient arylimines (entry 1–9, Table 2). However, when using imines pre-formed from 2-substituted benzaldehydes (entry 5, 6, Table 2) and naphthaldehydes (entry 11, 12, Table 2), reactions were rather slow with lower yields, perhaps owing to steric crowding. Not only aryl imines, but hetero-aryl imines also resulted in products with high yields (entry 13–16, Table 2), while the reactions with an alkenyl imine (entry 17, Table 2) and an in situ generated imine from formaldehyde (entry 18, Table 2) were sluggish, which resulted in low yields. In the case of electron-rich aryl imine and alkyl imine (entry 19 and 20, Table 2), the desired products were not obtained.
|
|
|||
|---|---|---|---|
| Entry | Product (4)a/time (h)b/yield (%)c | Entry | Product (4)a/time (h)b/yield (%)c |
| a (i) Imine 2 (0.3 mmol), 3 (3 M sol., 0.9 mmol), 1 (20 mol%), DMSO (3.0 mL), (ii) toluene (3.0 mL). b Time for Mannich reaction catalyzed by 1 (20 mol%). c Isolated yields refer to 2 (≤10% of aldehyde obtain in all the cases due to cleavage of imine). d Aldimines were prepared in situ. | |||
| 1 |
|
11 |
|
| 2 |
|
12 |
|
| 3 |
|
13 |
|
| 4 |
|
14 |
|
| 5 |
|
15 |
|
| 6 |
|
16 |
|
| 7 |
|
17 |
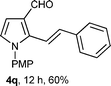
|
| 8 |
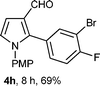
|
18d |
|
| 9 |
|
19 |
|
| 10 |
|
20d |
|
Based on our initial study and the literature precedents on proline catalyzed Mannich reactions, the following stepwise mechanism is proposed to account for this reaction. As shown in Scheme 1, the in situ generated enamine 5, generated from succinaldehyde 2 and proline 1, reacts with the N-PMP aldimine 3via a direct Mannich reaction to produce 6. The intermediate 6 undergoes intramolecular cyclization to hemiaminal 7 with the simultaneous regeneration of proline 1. Hemiaminal 7 underwent acid catalyzed dehydration and DDQ mediated aromatization to afford the substituted pyrrole 3-carboxaldehyde 4.
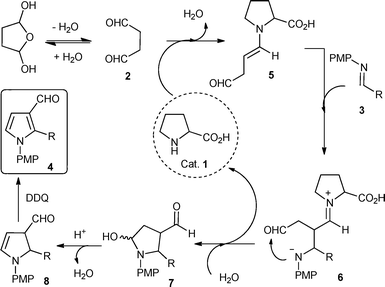 | ||
| Scheme 1 Mechanism for the two-step synthesis of pyrrole-3-carboxaldehyde 4. | ||
The presented two-step protocol also works very well at the preparative scale (2.0 mmol) of aldimines; resulting pyrroles 4 possessing the aldehyde functionality are important synthetic intermediates for further functionalization. A Wittig-reaction of 4j provided the corresponding α,β-unsaturated ester 9, and the trichloro-triazine (TCT) mediated dehydration17 of in situ generated oxime from 4j produced the corresponding nitrile compound 10 with high yields (Scheme 2).
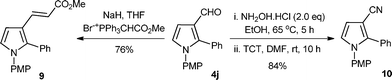 | ||
| Scheme 2 Synthetic transformation of pyrrole 3-carboxaldeyhde 4j. | ||
In conclusion, we have developed a new and direct method for the synthesis of substituted pyrrole-3-carboxaldehydes from readily available precursors such as N-PMP aldimines and succinaldehyde. The presented two-step protocol involves an organocatalytic Mannich reaction, followed by intramolecular cyclization and oxidative aromatization as a two-step sequence under mild conditions. In general, this method provides a new route to synthesizing substituted pyrroles from 1,4-dicarbonyl compounds, in addition to the Paal–Knorr condensation. Further applications of this methodology for the synthesis of densely substituted pyrroles is currently under investigation in our lab and will be presented in due course.
Acknowledgements
Authors acknowledge the financial support as a research initiation grant from BITS-Pilani. Instrumental analysis support from Dr Subrayashastry Aravinda (Scientist) and Deepika Singh (Scientist), IIIM (CSIR-Lab) Jammu, is also gratefully acknowledged.References
- (a) For reviews, see: J. S. Russel, E. T. Pelkey and S. J. P. Yoon-Miller, Prog. Heterocycl. Chem., 2011, 22, 143 CrossRef CAS; (b) B. A. Trofimov, A. I. Mikhaleva, E. Yu. Schmidt and L. N. Sobenina, Adv. Heterocycl. Chem., 2010, 99, 209 CrossRef CAS; (c) G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238 CrossRef CAS; (d) J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell Science, Oxford, 2000 Search PubMed; (e) R. A. Jones, The Chemistry of Heterocyclic Compounds: Pyrroles, Part I, Wiley, New York, 1990, Vol 48 Search PubMed.
- (a) D. L. Böger, C. W. Boyce, M. A. Labroli, C. A. Sehon and Q. Jin, J. Am. Chem. Soc., 1999, 121, 54 CrossRef; (b) C. T. Walsh, S. Garneau-Tsodikova and A. R. Howard-Jone, Nat. Prod. Rep., 2006, 23, 517 RSC; (c) M. Biava, Curr. Med. Chem., 2002, 9, 1859 CAS; (d) I. B. Seiple, S. Su, I. S. Young, A. Nakamura, A. Yamaguchi, L. Jørgensen, R. A. Rodriguez, D. P. O'Malley, T. Gaich, M. Köck and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 14710 CrossRef CAS; (e) H. Fan, J. Peng, M. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS; (f) J. H. Liu, Q. C. Yang, T. C. W. Mark and H. N. C. Wong, J. Org. Chem., 2000, 65, 3587 CrossRef CAS; (g) M. R. Rivero and S. L. Buchwald, Org. Lett., 2007, 9, 973 CrossRef CAS; (h) M. S. Butler, J. Nat. Prod., 2004, 67, 2141 CrossRef CAS; (i) I. S. Young, D. T. Paul and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801 RSC.
- (a) A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582 CrossRef; (b) M. Bialer, B. Yagen and R. Mechoulam, J. Med. Chem., 1979, 22, 1296 CrossRef CAS; (c) K. Starèević, M. Kralj, K. Ester, I. Sabol, M. Grce, K. Pavelić and G. Karminski-Zamola, Bioorg. Med. Chem., 2007, 15, 4419 CrossRef; (d) R. C. Arthur, T. J. Gupton, E. G. Kellogg, W. A. Yeudall, C. M. Cabot, I. F. Newsham and A. D. Gewirtz, Biochem. Pharmacol., 2007, 74, 981 CrossRef; (e) J. Lehuédé, B. Fauconneau, L. Barrier, M. Ourakow, A. Piriou and J.-M. Vierfond, Eur. J. Med. Chem., 1999, 34, 991 CrossRef; (f) I. Lessigiarska, A. Nankov, A. Bocheva, I. Pajeva and A. Bijev, Farmaco, 2005, 60, 209 CrossRef CAS; (g) S. Ushiyama, T. Yamada, Y. Murakami, S. Kumakura, S. Inoue, K. Suzuki, A. Nakao, A. Kawara and T. Kimura, Eur. J. Pharmacol., 2008, 578, 76 CrossRef CAS; (h) Y. Harrak, G. Rosell, G. Daidone, S. Plescia, D. Schillaci and M. D. Pujol, Bioorg. Med. Chem., 2007, 15, 4876 CrossRef CAS.
- (a) C. Della Pina, E. Falletta and M. Rossi, Catal. Today, 2011, 160, 11 CrossRef CAS; (b) C. F. Lee, L. M. Yang, T. Y. Hwu, A. S. Feng, J. C. Tseng and T. Y. Luh, J. Am. Chem. Soc., 2000, 122, 4992 CrossRef CAS; (c) S. K. Kim, J. L. Sessler, D. E. Gross, C.-H. Lee, J. S. Kim, V. M. Lynch, L. H. Delmau and B. P. Hay, J. Am. Chem. Soc., 2010, 132, 5827 CrossRef CAS; (d) X. Zhang, L. J. Richter, D. M. DeLongchamp, R. J. Kline, M. R. Hammond, I. McCulloch, M. Heeney, R. S. Ashraf, J. N. Smith, T. D. Anthopoulos, B. C. Schroeder, Y. H. Geerts, D. A. Fischer and M. F. Toney, J. Am. Chem. Soc., 2011, 133, 15073 CAS.
- (a) L. Knorr, Chem. Ber., 1884, 17, 1635 CrossRef; (b) C. Paal, Chem. Ber., 1885, 18, 367 CrossRef; (c) R. U. Braun, K. Zeitler and T. J. J. Muller, Org. Lett., 2001, 3, 3297 CrossRef CAS; (d) A. R. Bharadwaj and K. A. Scheidt, Org. Lett., 2004, 6, 2465 CrossRef CAS; (e) S. Werner, P. S. Iyer, M. D. Fodor, C. M. Coleman, L. A. Twining, B. Mitasev and K. M. Brummond, J. Comb. Chem., 2006, 8, 368 CrossRef CAS; (f) G. Minetto, L. F. Raveglia, A. Sega and M. Taddei, Eur. J. Org. Chem., 2005, 5277 CrossRef CAS; (g) J. Clayden, N. Greevs, S. Warren and P. Wothers, Organic Chemistry, Oxford University Press, Oxford, 2001, p. 1188 Search PubMed.
- (a) A. Padwa and W. H. Pearson, Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products, Wiley, New York, 2002, vol. 59 CrossRef; (b) S. Kamijo, C. Kanazawa and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 9260 CrossRef CAS; (c) N. C. Misra, K. Panda and H. Junjappa, J. Org. Chem., 2007, 72, 1246 CrossRef CAS; (d) Y. Kim, J. Kim and S. B. Park, Org. Lett., 2009, 11, 17 CrossRef CAS.
- (a) For reviews on multi-component reactions: V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2010, 39, 4402 RSC; (b) G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238 CrossRef CAS; (c) G. Balme, D. Bouyssi and N. Monteiro, Heterocycles, 2007, 73, 87 CrossRef CAS.
- (a) A. S. Dudnik and V. Gevorgyan, Catalysed Carbon-Heteroatom Bond Formation, ed. A. K. Yudin, Wiley-VCH, Weinheim, 2010, p. 227 Search PubMed; (b) Recent examples: S. Rakshit, F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9585 CrossRef CAS; (c) M. P. Huestis, L. Chan, D. R. Stuart and K. Fagnou, Angew. Chem., Int. Ed., 2011, 50, 1338 CrossRef CAS; (d) A. Mizuno, H. Kusama and N. Iwasawa, Angew. Chem., Int. Ed., 2009, 48, 8318 CrossRef CAS; (e) B. M. Trost, J.-P. Lumb and J. M. Azzarelli, J. Am. Chem. Soc., 2011, 133, 740 CAS; (f) M. Yamagishi, K. Nishigai, T. Hata and H. Urabe, Org. Lett., 2011, 13, 4873 CrossRef CAS; (g) Y. Jiang, W. C. Chan and C.-M. Park, J. Am. Chem. Soc., 2012, 134, 4104 CrossRef CAS.
- For indirect methods: (a) H. Jendralla, E. Baader, W. Bartmann, G. Beck, A. Bergmann, E. Granzer, B. Kerekjarto, K. Kesseler, R. Krause, W. Schubert and G. Wess, J. Med. Chem., 1990, 33, 61 CrossRef CAS; (b) A. R. Kelly, M. H. Kerrigan and P. J. Walsh;, J. Am. Chem. Soc., 2008, 130, 4097 CrossRef CAS; (c) B. L. Bray, P. H. Mathies, R. Naef, D. R. Solas, T. T. Tidwell, D. R. Artis and J. M. Muchowski, J. Org. Chem., 1990, 55, 6317 CrossRef CAS; (d) H. Dumoulin, F. Fabis, P. Dallemagne, S. Roult and M. Robba, Synth. Commun., 1994, 24, 1855 CrossRef; (e) For the direct method: A. Hamdan and J. W. F. Wasley, Synth. Commun., 1983, 13, 741 CrossRef CAS.
- (a) D. M. Collard and M. A. Fox, J. Am. Chem. Soc., 1991, 113, 9415 CrossRef; (b) C. P. Andrieux, P. Audebert, P. Hapiot and J. M. Saveant, J. Am. Chem. Soc., 1990, 112, 2439 CrossRef CAS; (c) H. Jendralla, E. Baader, W. Bartmann, G. Beck, A. Bergmann, E. Granzer, B. Kerekjarto, K. Kesseler, R. Krause, W. Schubert and G. Wess, J. Med. Chem., 1990, 33, 61 CrossRef CAS; (d) M. Kakushima, P. Hamel, R. Frenette and J. Rokach, J. Org. Chem., 1983, 48, 3214 CrossRef CAS; (e) H. J. Anderson and C. E. Loader, Synthesis, 1985, 353 CrossRef CAS.
- (a) For reviews on organocatalytic cascade reactions: Ł. Albrecht, H. Jiang and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 8492 CrossRef; (b) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS; (c) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS; (d) X. Yu and W. Wang, Org. Biomol. Chem., 2008, 6, 2037 RSC; (e) D. B. Ramachary and S. Jain, Org. Biomol. Chem., 2011, 9, 1277 RSC.
- (a) V. Nair, A. U. Vinod and C. Rajesh, J. Org. Chem., 2001, 66, 4427 CrossRef CAS; (b) R. Dhawan and B. A. Arndtsen, J. Am. Chem. Soc., 2004, 126, 468 CrossRef CAS; (c) D. J. St. Cyr and B. A. Arndtsen, J. Am. Chem. Soc., 2007, 129, 12366 CrossRef; (d) D. J. St. Cyr, N. Martin and B. A. Arndtsen, Org. Lett., 2007, 9, 449 CrossRef; (e) Y. Lu and B. A. Arndtsen, Org. Lett., 2009, 11, 1369 CrossRef CAS; (f) M. Shimizu, A. Takahashi and S. Kawai, Org. Lett., 2006, 8, 3585 CrossRef CAS.
- (a) I. Kumar, N. A. Mir, V. K. Gupta and Rajnikant, Chem. Commun., 2012, 48, 6975 RSC; (b) I. Kumar, N. A. Mir, C. V. Rode and B. P. Wakhloo, Tetrahedron: Asymmetry, 2012, 23, 225 CrossRef CAS; (c) I. Kumar and C. V. Rode, Tetrahedron: Asymmetry, 2010, 21, 2703 CrossRef CAS.
- See the ESI†.
- (a) Y. Hayashi, T. Okano, S. Aratake and D. Hazelard, Angew. Chem., Int. Ed., 2007, 46, 4922 CAS; (b) D. Hazelard, H. Ishikawa, D. Hashizume, H. Koshino and Y. Hayashi, Org. Lett., 2008, 10, 1445 CrossRef CAS.
- (a) A. Córdova and C. F. Barbas, III, Tetrahedron Lett., 2003, 44, 1923 CrossRef; (b) W. Notz, F. Tanaka, S. Watanabe, N. S. Chowdari, J. M. Turner, R. Thayumanavan and C. F. Barbas, III, J. Org. Chem., 2003, 68, 9624 CrossRef CAS; (c) Y.-C. Teo, J.-J. Lau and M.-C. Wu, Tetrahedron: Asymmetry, 2008, 19, 186 CrossRef CAS; (d) Y. Hayashi, T. Urushima, S. Aratake, T. Okano and K. Obi, Org. Lett., 2008, 10, 21 CrossRef CAS; (e) T. Hamada, K. Manabe and S. Kobayashi, Chem.–Eur. J., 2006, 12, 1205 CrossRef CAS; (f) T. Hamada, K. Manabe and S. Kobayashi, J. Am. Chem. Soc., 2004, 126, 7768 CrossRef CAS; (g) R.-G. Han, Y. Wang, Y.-Y. Li and P.-F. Xu, Adv. Synth. Catal., 2008, 350, 1474 CrossRef CAS.
- L. D. Luca, G. Giacomelli and A. Porcheddu, J. Org. Chem., 2002, 67, 6272 CrossRef.
Footnotes |
| † Electronic Supplementary Information (ESI) available: For experimental procedures and characterization data of new compounds. See DOI: 10.1039/c2ra21258g |
| ‡ This article is dedicated to Prof. H. Ila for her contribution to heterocyclic chemistry. |
| This journal is © The Royal Society of Chemistry 2012 |