Solvent free L-proline-catalysed domino Knoevenagel/6π-electrocyclization for the synthesis of highly functionalised 2H-pyrans†
Javier
Peña
,
Rosalina F.
Moro
,
P.
Basabe
,
Isidro S.
Marcos
and
David
Díez
*
Departamento Química Orgánica, Facultad de Ciencias Químicas, Universidad de Salamanca, Salamanca, Spain. E-mail: ddm@usal.es; Fax: 0034923294574; Tel: 0034923294500. Ext. 1529
First published on 4th July 2012
Abstract
An environmentally benign synthesis of 2H-pyrans has been achieved in high yield using β-oxosulfones and 3,3-dialkylsubstituted α,β-unsaturated aldehydes. The key reaction is a solvent free L-proline catalysed domino Knoevenagel/6π-electrocyclization. As 3,3-dialkylsubstituted α,β-unsaturated aldehydes are a very common feature in many natural products, the transformation into highly functionalised 2H-pyrans makes this procedure a green and excellent method for a diversity oriented synthesis of biologically active compounds.
Introduction
Molecules with a pyran heterocycle in their structure are very interesting due to their biological activities and applications in medicine.1 Benzopyrans, such as daurichromenic acid, an anti-HIV agent,2 seselin, an antinociceptive along with other activities,3 or privileged structures, such as 2-pyrones, an essential pharmacophore in many naturally occurring and biologically active compounds,4 led our attention to the 2H-pyran ring. Although these compounds are very interesting, there is not a great variety of starting materials to synthesise them, being usually made by iminium activation of a carbonyl group and a 1,3-dicarbonyl compound.5 As an example, Profs. Chang and Marsella et al. described the reaction of the E/Z mixture of citral with 1,3-cyclohexanodione to yield perhydro-CBC (cannabichromene), which was later transformed into a Δ1-tetrahydrocannabinol analogue6 (Fig. 1). Prof. Jørgensen et al. have reported other very interesting uses of β-ketoesters in organocatalytic conditions.6b,c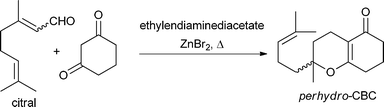 | ||
| Fig. 1 Synthesis of perhydro-CBC by Profs. Chang and Marsella. | ||
In our group we have been interested in the reactivity of β-oxosulfones, which are known substrates in organocatalysis,7 for the synthesis of 2-alkylidene cyclohexenones by a Michael addition8 using organocatalyst 5 followed by a Morita–Baylis–Hillman reaction after addition of L-proline (Scheme 1). The use of β-oxosulfones over 1,3-dicarbonyl compounds will add the extra versatility of the sulfone moiety, mainly due to easy elimination and reactivity.
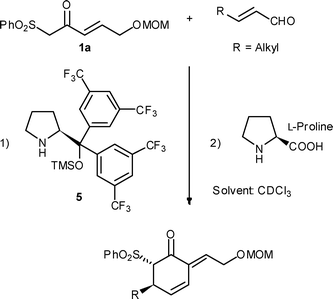 | ||
| Scheme 1 Synthesis of 2-alkylidene cyclohexenones. | ||
Prof. Inokuchi et al. established that 2-alkylsubstituted enals favour the formation of (E)-Knoevenagel adducts for the ensuing electrocyclization.4 We became interested in carrying out the reaction with our β-oxosulfones and 3,3-dialkylsubstituted enals, because of its profusion in nature,9 as in the case of Chang and Marsella,6 using organocatalysts in order to develop environmentally benign processes for the synthesis of 2H-pyrans. We were also wondering if it would be possible to obtain the corresponding pyrans by changing the mechanism of the reaction between Nazarov reagents such as 1a and 3,3-dialkylsubstituted α,β-unsaturated aldehydes.
L-Proline has been, since the leading paper of List, Lerner and Barbas,10 one of the most widely employed organocatalysts, not only for simple reactions such as the aldol,11 Mannich,12 Michael,13 Biginelli,14 Diels–Alder/Knoevenagel,15 Baylis–Hillman,16 aza-Morita–Baylis–Hillman,17 α-selenylation,18 α-halogenation19 and oxidation20 reactions, among others, but also in tandem or multicomponent reactions.21 Proline is a very cheap, nontoxic amino acid available in both enantiomeric forms, from which many derivatives have been developed for its use in organocatalysis,22 and ideal for green chemistry.21a,b This is an area of growing interest in recent years, as better procedures with less toxic solvents and no hazardous chemicals should be the aim of any chemist.23
Herein we report, to the best of our knowledge, the first synthesis of highly substituted 2H-pyrans using an efficient, practical and environmentally friendly methodology under solvent-free conditions, using L-proline as a catalyst.
Results and discussions
The key strategy is the reaction of a β-oxosulfone with 3,3-disubstituted enals.24 First of all we decided to screen different catalysts and additives (Table 1 and Fig. 2) in the reaction between β-oxosulfone 1a and the E/Z mixture of citral in isopropyl alcohol, as it was the best solvent used in our previous studies for the synthesis of 2-alkylidene cyclohexenones.8 | ||
| Fig. 2 Catalysts and additives for use in the synthesis of 2H-pyrans. | ||
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Additive | Timec | Yieldd (%) | |
| 2 | 3a | ||||
a All the reactions were carried out at r.t., in iPrOH at 0.18 M, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of sulfone to E/Z-citral, 20 mol% catalyst and 20 mol% additive.
b S.M. = starting materials; B.A. = benzoic acid; 12 = BinapPOH = (S)-(+)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate; 13 = CF3-BinapPOH = (R)-3,3′-bis[3,5-bis(trifluoromethyl)phenyl]-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate.
c Time in which highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
d Isolated yield after chromatography on silica gel.
e Benzoic acid was added 6 days after catalyst 4.
f Benzoic acid was added 6 days after catalyst 5.
g Catalyst 5 was added 10 h after BinapPOH.
h
L-Proline was added 26 h after catalyst 5.
i Benzoic acid was added 6 days after catalyst 10. 1 ratio of sulfone to E/Z-citral, 20 mol% catalyst and 20 mol% additive.
b S.M. = starting materials; B.A. = benzoic acid; 12 = BinapPOH = (S)-(+)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate; 13 = CF3-BinapPOH = (R)-3,3′-bis[3,5-bis(trifluoromethyl)phenyl]-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate.
c Time in which highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
d Isolated yield after chromatography on silica gel.
e Benzoic acid was added 6 days after catalyst 4.
f Benzoic acid was added 6 days after catalyst 5.
g Catalyst 5 was added 10 h after BinapPOH.
h
L-Proline was added 26 h after catalyst 5.
i Benzoic acid was added 6 days after catalyst 10.
|
|||||
| 1 | DABCO | — | 21 h | S.M. | |
| 2 | DBU | — | 3 d | S.M. | |
| 3 | Et3N | — | 3 d | S.M. | |
| 4 | Piperidine | — | 3 d | Decomposition | |
| 5 | Pyrrolidine | — | 2 h | Decomposition | |
| 6 | Pyridine | — | 3 d | — | 16 |
| 7 | — | B.A. | 45 d | <5 | — |
| 8 | — | 12 | 10 h | S.M. | |
| 9 | — | 13 | 12 h | S.M. | |
| 10 | 11 | B.A. | 8 d | S.M. | |
| 11 | L-Proline | — | 6 h | 11 | 63 |
| 12 | L-Proline | LiOAc | 13 h | 29 | 44 |
| 13 | L-Proline | B.A. | 17 h | 9 | 62 |
| 14 | L-Proline | 12 | 24 h | 4 | 96 |
| 15 | L-Proline | 13 | 17 h | 1 | 99 |
| 16e | 4 | B.A. | 8 d | S.M. | |
| 17f | 5 | B.A. | 8 d | S.M. | |
| 18g | 5 | 12 | 60 d | — | 15 |
| 19fh | 5 + L-Proline | LiOAc | 39 h | 10 | — |
| 20fh | 5 + L-Proline | B.A. | 39 h | 7 | 33 |
| 21 | 6 | — | 6 d | — | 60 |
| 22 | 7 | — | 13 h | — | 33 |
| 23 | 8 | — | 25 d | S.M. | |
| 24 | 9 | — | 10 d | — | 11 |
| 25i | 10 | B.A. | 8 d | S.M. | |
We first used the usual bases to see if the Knoevenagel reaction proceeded as the first step, but these led to low yields, decomposition or simply no reaction after several days (Table 1, entries 1–6). Benzoic acid or even chiral acids, i.e.12 and 13,25 were tested, as they can be used as additives, and thioureas, i.e.11, were also tested, but the reaction either did not proceed at all or did not proceed in a reasonable time (entries 7–10). Only the citral dimer 2 was formed in very low yield after 45 days with benzoic acid, previously obtained by Watanabe et al. in the reaction of citral with proline26 (entry 7). Then several organocatalysts (Fig. 2) were tested, with and without additives. When L-proline was used, the yield of 2H-pyran 3a increased dramatically, especially when acid 13 was used as an additive (entries 11–15). Other organocatalysts did not give any good results with or without additives (entries 16–25).
As L-proline was found to be the best organocatalyst, we then screened different solvents (Table 2). From this study, solvent free conditions with no additive turned out to be the best conditions (entry 10). Surprisingly, using additive 13 did not lead to any increase in the yield, but suppressed the production of the citral dimer in no solvent conditions (Table 2, entries 11 and 12). In all cases racemic mixtures were obtained, ascertained by HPLC and optical rotatory power, even when additives 12 or 13 were used in order to induce chirality. Although the yield was excellent when isopropyl alcohol and additive 13 were used (Table 1, entry 15) the use of no solvent and no expensive additives makes this procedure to obtain 2H-pyrans easier and more environmentally efficient than the previous ones. In the case of reagent solubility problems, the use of isopropyl alcohol is a green alternative too. These conditions are not exclusive to β-oxosulfones and can be applied to 1,3-diketones, β-ketoesters or β-ketoamides.
| Entry | Solvent | Timeb (h) | Yieldc (%) | |
|---|---|---|---|---|
| 2 | 3a | |||
a All the reactions were carried out at r.t., at 0.18 M, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of sulfone to E/Z-citral, 20 mol% L-proline and 20 mol% 12.
b Time in which the highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel.
d S.M. = starting materials.
e No additives used.
f 20 mol % 13 added to the reaction as additive instead of 12. 1 ratio of sulfone to E/Z-citral, 20 mol% L-proline and 20 mol% 12.
b Time in which the highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel.
d S.M. = starting materials.
e No additives used.
f 20 mol % 13 added to the reaction as additive instead of 12.
|
||||
| 1 | Hexane | 54 | 12 | 72 |
| 2 | Toluene | 29 | — | 32 |
| 3 | CHCl2 | 29 | 13 | 34 |
| 4 | CHCl3 | 29 | — | 42 |
| 5 | Diethyl ether | 29 | — | 37 |
| 6 | THF | 29 | — | 20 |
| 7 | MeOH | 8 | — | 28 |
| 8 | EtOH | 8 | — | 39 |
| 9 | H2O | 56 | S.M.d | |
| 10 e | NO SOLVENT | 22 | 13 | 87 |
| 11 | NO SOLVENT | 24 | — | 58 |
| 12f | NO SOLVENT | 21 | — | 49 |
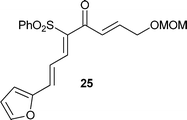
Having established the best conditions, we decided to evaluate our method with different aldehydes, using firstly the same starting material, the β-oxosulfone 1a, as shown in Table 3. In all cases where the unsaturated aldehyde was 3,3-dialkylsubstituted, the 2H-pyran was obtained in a very good yield but as a racemic mixture, ascertained by HPLC and optical rotatory power, entries 1–6, or even in the case of entry 7. In the case of compounds 18 and 20, entries 5 and 6, the corresponding diastereoisomeric mixtures were obtained due to the presence of epimers at the tetrasubstituted carbon of the pyran ring. These results can be explained by the proposed mechanism (Fig. 3): initial Knoevenagel condensation leads to intermediate A, which after 6π-electrocyclization produces the corresponding 2H-pyran.27 Other aldehydes, with a different substitution pattern, only gave the corresponding Knoevenagel products (entries 8–10), whose stereochemistry was established by bidimensional NMR and NOE experiments. A similar result was obtained by Profs. Mischne and Riveira in polycyclization reactions of 1,3-dicarbonyl compounds and α,β,γ,δ-unsaturated aldehydes, where the unsubstituted one gives only condensation and no cyclization.28 Of special interest are the compounds obtained in entries 5 and 6, analogues of marine natural products, such as the pyranocoumarin ferprenin.29 As there are many natural products with a 3,3-dialkylsubstituted α,β-unsaturated aldehyde functionality,9 this procedure opens up an environmentally acceptable method for the synthesis of 2H-pyrans for diversity oriented synthesis.30
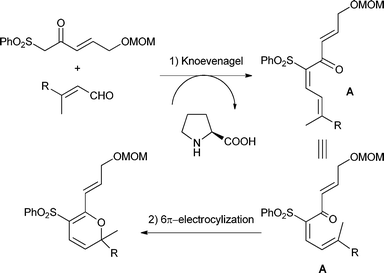 | ||
| Fig. 3 Proposed mechanism for the formation of 2H-pyrans. | ||
| Entry | α,β-Unsaturated aldehyde | Timeb | Product | Yieldc (%) |
|---|---|---|---|---|
a All the reactions were carried out at r.t., in iPrOH at 0.18 M, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of sulfone 1a to aldehyde and 20 mol% L-proline or under solvent free conditions. In all cases the yields were similar.
b Time in which highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel.
d 20 mol% of BinapPOH, 12, added to the reaction. 1 ratio of sulfone 1a to aldehyde and 20 mol% L-proline or under solvent free conditions. In all cases the yields were similar.
b Time in which highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel.
d 20 mol% of BinapPOH, 12, added to the reaction.
|
||||
| 1 |

|
4.5 h |

|
82 |
| 2 |

|
6 h |

|
63 |
| 3 |

|
15 h |

|
58 |
| 4d |

|
15 h |

|
58 |
| 5 |

|
7 h |

|
38 |
| 6 |
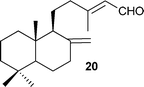
|
3 d |

|
63 |
| 7 |

|
5 d |
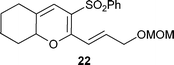
|
43 |
| 8 |

|
21 h |
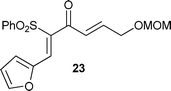
|
87 |
| 9 |
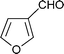
|
1 d |

|
99 |
| 10 |
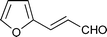
|
15 h |
|
56 |
Once the procedure for the synthesis of 2H-pyrans had been carried out consistently with different aldehydes, it was subjected to a study with other β-oxosulfones in order to define the scope and consistency of the reaction (Table 4).
| Entry | β-oxosulfone | Product | Timeb (h) | Yieldc (%) | |
|---|---|---|---|---|---|
| 2 | 3(b,c) | ||||
a All the reactions were carried out at r.t., in iPrOH at 0.18 M, or under free solvent conditions with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of sulfone to E/Z-citral, and 20 mol% L-proline. In all cases the yield was similar, except for compound 1c which, being a solid, was reacted in iPrOH only.
b Time in which the highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel. 1 ratio of sulfone to E/Z-citral, and 20 mol% L-proline. In all cases the yield was similar, except for compound 1c which, being a solid, was reacted in iPrOH only.
b Time in which the highest yield was observed with no decomposition (the consumption of starting materials was monitored by TLC).
c Isolated yield after chromatography on silica gel.
|
|||||
| 1 |

|

|
7 | 3 | 97 |
| 2 |

|
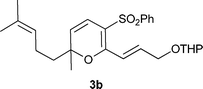
|
5 | 12 | 88 |
| 3 |

|

|
15 | 36 | 64 |
The synthesis of these β-oxosulfones is shown in Scheme 2. After monoprotection under standard conditions of the commercially available E-1,4-butenediol with 3,4-dihydro-2H-pyran (DHP) gave 26,31 which was oxidised with pyridinium dichromate (PDC) and condensed with the lithium derivative of methylphenylsulfone, leading to the corresponding alcohol 28, whose oxidation gave the corresponding mixture of β-oxosulfones 1b and 1c in a 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Use of other oxidising conditions, including the use of catalytic tetrapropylammonium perruthenate (TPAP), led to a decrease in the yield of the oxidation steps. These sulfones were separated by flash chromatography on silica gel and deprotection of the major compound gave the β-oxosulfone 1d. As shown in Table 4, the three sulfones 1b, 1c, and 1d behave exactly as before, yielding the corresponding 2H-pyrans when reacted with E/Z-citral, making this procedure ideal for the synthesis of highly functionalised 2H-pyrans in environmentally safe conditions. The presence of the sulfone group in these compounds adds an extra functionality and so more versatility for further research.
1 ratio. Use of other oxidising conditions, including the use of catalytic tetrapropylammonium perruthenate (TPAP), led to a decrease in the yield of the oxidation steps. These sulfones were separated by flash chromatography on silica gel and deprotection of the major compound gave the β-oxosulfone 1d. As shown in Table 4, the three sulfones 1b, 1c, and 1d behave exactly as before, yielding the corresponding 2H-pyrans when reacted with E/Z-citral, making this procedure ideal for the synthesis of highly functionalised 2H-pyrans in environmentally safe conditions. The presence of the sulfone group in these compounds adds an extra functionality and so more versatility for further research.
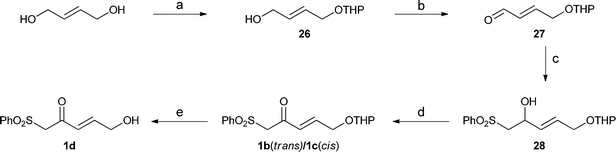 | ||
Scheme 2 Reagents and conditions for the synthesis of β-oxosulfones 1b, 1c and 1d: (a) DHP, pTsOH (1%), DCM, r.t., 96%; (b) PDC (2 equiv.), molecular sieves, DCM, r.t., 91%; (c) methylphenylsulfone (0.9 equiv.), n-BuLi (0.9 equiv.), THF, −78 °C, 61%; (d) PDC (2 equiv.), molecular sieves, r.t., 58%, (ratio 1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c = 99 1c = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); and (e) pTsOH (10%), THF–H2O (1 1); and (e) pTsOH (10%), THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), r.t., 88%. 1), r.t., 88%. | ||
Conclusions
We have established a new and simple procedure for the synthesis of 2H-pyrans. This method shows once again the versatility of L-proline as a catalyst, since it is the only one that gives the reaction. The reaction proceeds under solvent free conditions, becoming a green way to obtain compounds of high impact for the synthesis of natural compound analogues with biological activity in diversity oriented synthesis.Experimental section
Unless otherwise stated, all chemicals were purchased as the highest purity commercially available and were used without further purification, except for 1a,8 10-hydroxycitral 15,32 farnesal,33189c and 20,9d,f which were synthesised according to the literature procedures. For more details see the ESI.†1. Synthesis of the Nazarov reagents (1b–1d)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) afforded 28 (4.63 g, 61%). υmax (liquid film) 3444, 2953, 2872, 2250, 1732, 1446, 1288, 1138 δH (200 MHz; CDCl3) 7.97–7.83 (2H, m, ArHortho), 7.71–7.46 (3H, m, ArHmeta, ArHpara), 5.83 (1H, dt, J = 15.5, 5.2 Hz, H4), 5.62 (1H, dd, J = 15.5, 5.2 Hz, H3), 4.76–4.59 (1H, m, H7), 4.59–4.49 (1H, m, H2), 4.15 (1H, dd, J = 13.3, 4.6 Hz, H5a), 3.95–3.69 (2H, m, H1a and H5a), 3.55–3.41 (2H, m, H9), 3.30–3.19 (2H, m, H1b and 5b), 1.84–1.37 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 139.7, 134.1, 131.4, 129.5 (2C), 128.8, 128.1 (2C), 98.1, 66.6, 66.5, 62.2, 62.1, 30.6, 25.5, 19.5; EIHRMS: Calcd for C16H22O5S (M + Na): 349.1080; found: 349.1080 (M + Na).
4) afforded 28 (4.63 g, 61%). υmax (liquid film) 3444, 2953, 2872, 2250, 1732, 1446, 1288, 1138 δH (200 MHz; CDCl3) 7.97–7.83 (2H, m, ArHortho), 7.71–7.46 (3H, m, ArHmeta, ArHpara), 5.83 (1H, dt, J = 15.5, 5.2 Hz, H4), 5.62 (1H, dd, J = 15.5, 5.2 Hz, H3), 4.76–4.59 (1H, m, H7), 4.59–4.49 (1H, m, H2), 4.15 (1H, dd, J = 13.3, 4.6 Hz, H5a), 3.95–3.69 (2H, m, H1a and H5a), 3.55–3.41 (2H, m, H9), 3.30–3.19 (2H, m, H1b and 5b), 1.84–1.37 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 139.7, 134.1, 131.4, 129.5 (2C), 128.8, 128.1 (2C), 98.1, 66.6, 66.5, 62.2, 62.1, 30.6, 25.5, 19.5; EIHRMS: Calcd for C16H22O5S (M + Na): 349.1080; found: 349.1080 (M + Na).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) afforded 1b (304 mg, 57%) and 1c (5 mg, 1%). 1b: υmax (liquid film) 2943, 2870, 2852, 1693, 1666, 1633, 1446, 1384, 1325, 1153; δH (200 MHz; CDCl3) 7.89 (2H, d, J = 7.0 Hz, ArHortho), 7.74–7.50 (3H, m, ArHmeta and ArHpara), 6.98 (1H, dt, J = 15.8, 3.9 Hz, H4), 6.54 (1H, dt, J = 15.8, 1.9 Hz, H3), 4.65 (1H, t, J = 3.1 Hz, H7), 4.46 (1H, ddd, J = 17.6, 3.9, 1.9 Hz, H5a), 4.32 (2H, s, H1), 4.18 (1H, ddd, J = 17.6, 3.9, 2.0 Hz, H5b), 3.89–3.74 (1H, m, H9a), 3.59–3.42 (1H, m, H9b), 1.90–1.40 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 187.3, 147.7, 138.9, 134.4, 129.4 (2C), 128.6 (2C), 128.0, 98.4, 65.6, 65.3, 62.2, 30.5, 25.5, 19.3; EIHRMS: Calcd for C16H20O5S (M + Na): 347.0924; found: 347.0924 (M + Na). 1c: υmax (liquid film) 2943, 2872, 2852, 1693, 1666, 1614, 1448, 1377, 1323, 1155; δH (200 MHz; CDCl3) 8.01–7.80 (2H, m, ArHortho), 7.79–7.50 (3H, m, ArHmeta and ArHpara), 6.65–6.37 (2H, m, H3 and H4), 4.75–4.39 (3H, m, H5 and H7), 4.21 (2H, s, H1), 3.92–3.71 (1H, m, H9a), 3.58–3.38 (1H, m, H9b), 1.95–1.36 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 187.4, 152.4, 138.8, 134.5, 129.6 (2C), 128.5 (2C), 124.7, 99.2, 68.1, 67.2, 62.8, 30.8, 25.6, 19.8; EIHRMS: Calcd for C16H20O5S (M + Na): 347.0924; found: 347.0924 (M + Na).
4) afforded 1b (304 mg, 57%) and 1c (5 mg, 1%). 1b: υmax (liquid film) 2943, 2870, 2852, 1693, 1666, 1633, 1446, 1384, 1325, 1153; δH (200 MHz; CDCl3) 7.89 (2H, d, J = 7.0 Hz, ArHortho), 7.74–7.50 (3H, m, ArHmeta and ArHpara), 6.98 (1H, dt, J = 15.8, 3.9 Hz, H4), 6.54 (1H, dt, J = 15.8, 1.9 Hz, H3), 4.65 (1H, t, J = 3.1 Hz, H7), 4.46 (1H, ddd, J = 17.6, 3.9, 1.9 Hz, H5a), 4.32 (2H, s, H1), 4.18 (1H, ddd, J = 17.6, 3.9, 2.0 Hz, H5b), 3.89–3.74 (1H, m, H9a), 3.59–3.42 (1H, m, H9b), 1.90–1.40 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 187.3, 147.7, 138.9, 134.4, 129.4 (2C), 128.6 (2C), 128.0, 98.4, 65.6, 65.3, 62.2, 30.5, 25.5, 19.3; EIHRMS: Calcd for C16H20O5S (M + Na): 347.0924; found: 347.0924 (M + Na). 1c: υmax (liquid film) 2943, 2872, 2852, 1693, 1666, 1614, 1448, 1377, 1323, 1155; δH (200 MHz; CDCl3) 8.01–7.80 (2H, m, ArHortho), 7.79–7.50 (3H, m, ArHmeta and ArHpara), 6.65–6.37 (2H, m, H3 and H4), 4.75–4.39 (3H, m, H5 and H7), 4.21 (2H, s, H1), 3.92–3.71 (1H, m, H9a), 3.58–3.38 (1H, m, H9b), 1.95–1.36 (6H, m, H10, H11, and H12); δC (50 MHz; CDCl3) 187.4, 152.4, 138.8, 134.5, 129.6 (2C), 128.5 (2C), 124.7, 99.2, 68.1, 67.2, 62.8, 30.8, 25.6, 19.8; EIHRMS: Calcd for C16H20O5S (M + Na): 347.0924; found: 347.0924 (M + Na).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF–H2O, and the whole mixture was stirred for 5 days. The reaction was quenched with H2O, extracted with EtOAc and the combined organics were washed with NaHCO3 (5%), H2O and brine, dried (Na2SO4), filtered and concentrated in vacuo to afford 1d (130.2 mg, 88%). υmax (liquid film) 3504, 2931, 1691, 1664, 1627, 1448, 1309, 1151; δH (200 MHz; CDCl3) 7.93–7.82 (2H, m, ArHortho), 7.72–7.50 (3H, m, ArHmeta and ArHpara), 7.04 (1H, dt, J = 15.8, 3.6 Hz, H4), 6.58 (1H, dt, J = 15.8, 2.0 Hz, H3), 4.38 (2H, m, H5), 4.33 (2H, s, H1); δC (50 MHz; CDCl3) 187.2, 150.4, 138.8, 134.6, 129.6 (2C), 128.6 (2C), 127.1, 65.8, 62.0; EIHRMS: Calcd for C11H12O4S (M + Na): 263.0349; found: 263.0349 (M + Na).
1 mixture of THF–H2O, and the whole mixture was stirred for 5 days. The reaction was quenched with H2O, extracted with EtOAc and the combined organics were washed with NaHCO3 (5%), H2O and brine, dried (Na2SO4), filtered and concentrated in vacuo to afford 1d (130.2 mg, 88%). υmax (liquid film) 3504, 2931, 1691, 1664, 1627, 1448, 1309, 1151; δH (200 MHz; CDCl3) 7.93–7.82 (2H, m, ArHortho), 7.72–7.50 (3H, m, ArHmeta and ArHpara), 7.04 (1H, dt, J = 15.8, 3.6 Hz, H4), 6.58 (1H, dt, J = 15.8, 2.0 Hz, H3), 4.38 (2H, m, H5), 4.33 (2H, s, H1); δC (50 MHz; CDCl3) 187.2, 150.4, 138.8, 134.6, 129.6 (2C), 128.6 (2C), 127.1, 65.8, 62.0; EIHRMS: Calcd for C11H12O4S (M + Na): 263.0349; found: 263.0349 (M + Na).
2. General procedure for the synthesis of 2H-pyrans (3a–3c)
β-Oxosulfone (1a–1d) (17.6 mmol) and E/Z-citral (8.7 mmol) were dissolved in 1 ml isopropyl alcohol. Next, L-proline (20 mol%), and an additive (20 mol%) if needed, were added and left stirring for the appropriate time. All products were purified by flash chromatography on silica gel using different mixtures of hexane–EtOAc.3. General procedure for the synthesis of 2H-pyrans and Knoevenagel adducts
β-Oxosulfone 1a (50 mg, 17.6 mmol) and the corresponding aldehyde (8.7 mmol) were dissolved in 1 ml isopropyl alcohol. Next, L-proline (20 mol%), and an additive (20 mol%) if needed were added and left stirring for the appropriate time. All products were purified by flash chromatography on silica gel using different mixtures of hexane–EtOAc.[α]D = +35.4 (c 1.07, CHCl3); υmax (liquid film) 2932, 2874, 1726, 1539, 1446, 1321, 1157; δH (400 MHz; CDCl3) 7.86 (2H, d, J = 7.3 Hz, ArHortho), 7.59–7.41 (3H, m, ArHmeta, ArHpara), 7.41 (1H, m, H1′), 6.59 (1H, m, H2′), 6.37 (1H, dd, J = 9.8, 3.0 Hz, H4), 5.33 (1H, d, J = 9.8 Hz, H3), 5.29–5.17 (1H, m, H9′′), 4.68 (2H, s, O–CH2–O), 4.26 (2H, d, J = 5.2, Hz, H3′), 3.61 (3H, s, COO–CH3), 3.40 (3H, s, O–CH3), 2.65–2.50 (1H, m, H5′′), 1.97–0.65 (25H, m, H1′′, H2′′, H6′′, H7′′, H8′′, H10′′, H11′′, H13′′, H14′′, H16′′, H1′′′); δC (101 MHz; CDCl3) 178.3, 156.3, 143.1, 141.1, 135.9, 132.5, 129.0 (2C), 126.4 (2C), 124.8, 121.5, 119.8, 119.1, 114.3, 96.0, 80.9, 67.1, 55.3, 51.6, 44.8, 42.7, 38.3(2C), 34.6, 31.4, 30.6, 28.6, 26.2, 22.9, 22.8, 22.3, 19.9, 15.4; EIHRMS: Calcd for C34H46O7S (M + Na): 621.2856; found: 621.2856 (M + Na).
[α]D = +4.58 (c 0.3, CHCl3); υmax (liquid film) 2918, 2848, 1539, 1446, 1321, 1153; δH (200 MHz; CDCl3) 7.85 (2H, dd, J = 7.6, 1.6 Hz, ArHortho), 7.60–7.47 (3H, m, ArHmeta, ArHpara), 7.40 (1H, m, H1′), 6.56 (1H, dt, J = 10.1, 5.4 Hz, H2′), 6.36 (1H, dd, J = 10.1, 1.3 Hz, H4), 5.34 (1H, d, J = 10.1, H3), 4.74 (1H, s, H29′′a), 4.68 (2H, s, O–CH2–O), 4.38 (1H, s, H29′′b), 4.25 (2H, d, J = 5.4, Hz, H3′), 3.40 (3H, s, O–CH3), 2.44–0.87 (28H, m, H1′′, H2′′, H3′′, H5′′, H6′′, H7′′, H9′′, H10′′, H11′′, H13′′, H15′′, H16′′, H1′′′); δC (50 MHz; CDCl3) 156.3, 148.5, 143.4, 136.4, 132.8, 129.3 (2C), 126.7 (2C), 124.6, 121.8, 119.5, 114.5, 106.6, 96.2, 81.3, 67.2, 57.3, 55.7, 55.6, 42.4, 40.1, 40.0, 39.1, 38.5, 33.8, 33.5, 26.2, 24.6, 21.9, 19.6, 19.2, 14.6; EIHRMS: Calcd for C33H46O5S (M + Na): 577.2958; found: 577.2958 (M + Na).
Acknowledgements
The authors gratefully acknowledge the help of A. Lithgow (NMR) and C. Raposo (MS) of Universidad de Salamanca and FSE, MICINN CTQ2009-11172BQU, Junta de Castilla and León for financial support. J.P. is grateful to the MICINN for its fellowship.References
- (a) D. Yang, L. Shi, H. Huang, J. Zhang and L. Gan, J. Org. Chem., 2010, 75, 4567 CrossRef CAS; (b) I. Larrosa, P. Romea and F. Urpí, Tetrahedron, 2008, 64, 2683 CrossRef CAS.
- (a) Y. R. Lee, J. H. Choi and S. H. Yoon, Tetrahedron Lett., 2005, 46, 7539 CrossRef CAS; (b) Y. Kashiwada, K. Yamazaki, Y. Ikeshiro, T. Yamagishi, T. Fujioka, K. Mihashi, K. Mizuki, L. M. Cosentino, K. Fowke, S. L. Morrish-Natschke and K-H. Lee, Tetrahedron, 2001, 57, 1559 CrossRef CAS; (c) See also the excellent review on privileged structures or privileged substructures: D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS; (d) For a environmentally benign synthesis of pyrans see: F. J. Jung, B. H. Park and Y. R. Lee, Green Chem., 2010, 12, 2003 RSC.
- (a) K. C. Nicolaou, J. A. Pfefferrkorn and G-Q. Cao, Angew. Chem., Int. Ed., 2000, 39, 734 CrossRef CAS; (b) V. Lima, C. B. Silva, J. Mafezoli, M. Bezerra, M. O. Moraes, G. S. M. M. Mourao, J. N. Silva and M. C. F. Oliveira, Fitoterapia, 2006, 77, 574 CrossRef CAS.
- (a) W. Peng, T. Hirabaru, H. Kawafuchi and T. Inokuchi, Eur. J. Org. Chem., 2011, 5469 CrossRef CAS and references cited therein; (b) H. Leitbecher, J. Conrad, I. Klaiber and U. Beifuss, QSAR Comb. Sci., 2004, 23, 895 CrossRef; (c) H. Leutbecher, S. Rieg, J. Conrad, S. Mika, I. Klaiber and U. Beifuss, Z. Naturfsoch., 2009, 64b, 935 Search PubMed.
- (a) For leading references on oxa-[3 + 3] strategy see: G. S. Buchanan, K. P. Cole, G. Li, Y. Tang, L-F. You and R. P. Hsung, Tetrahedron, 2011, 67, 10105 CrossRef CAS; (b) G. S. Buchanan, K. P. Cole, Y. Tangand and R. P. Hsung, J. Org. Chem., 2011, 76, 7027 CrossRef CAS; (c) Y. Tang, J. Oppenheimer, Z. Song, L. You, X Zhang and R. P. Hsung, Tetrahedron, 2006, 62, 10785 CrossRef CAS; (d) See also: Y. R. Lee, D. H. Kim, J-J. Shim, S. K. Kim, J. H. Park, J. S. Cha and C-S. Lee, Bull. Korean Chem. Soc., 2002, 23, 998 CrossRef CAS; (e) X-Y. Tnag, Y. Wei and M. Shi, Org. Lett., 2010, 12, 5120 CrossRef; (f) M. S. Aingh, G. C. Nandi and S. Samai, Green Chem., 2012, 14, 447 RSC; (g) C. Arróniz, C. Escolano, F. J. Luque, J. Bosch and M. Amat, Org. Biomol. Chem., 2011, 9, 5079 RSC.
- (a) A. García, D. Borchardt, C-E. A. Chang and M. J. Marsella, J. Am. Chem. Soc., 2009, 131, 16640 CrossRef; (b) S. Cabrera, J. Alemán, P. Bolze, S. Bertelsend and K. A. Jørgensen, Angew. Chem., Int. Ed., 2008, 47, 121 CrossRef CAS; (c) L. Albrecht, B. Richter, C. Vila, H. Krawcyzk and K. A. Jørgensen, Chem.–Eur. J., 2009, 13, 3093 CrossRef; (d) See also the excellent review on the application of the formal [3 + 3] cycloaddition to Natural Product Synthesis: R. P. Hsung, A. V. Kurdyumov and N. Sydorenko, Eur. J. Org. Chem., 2005, 1, 23 CrossRef.
- (a) J. Alemán, V. Marcos, L. Marzo and J. L. G. Ruano, Eur. J. Org. Chem., 2010, 4482 Search PubMed; (b) For use of β-ketosulfoxides see: J. L. Ruano, C. Alvarado, S. Díaz-Tendero and J. Alemán, Chem. Eur. J., 2009, 13, 3093 Search PubMed; (c) For a review on sulfones in organocatalysis, see: M. Nielsen, C. Jacobsen, N. Holub, M. Paixão and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 2668 CrossRef CAS; (d) See also: M. Nielsen, C. B. Jacobsen, M. W. Paixão, N. Holub and K. A. Jørgensen, J. Am. Chem. Soc., 2009, 131, 10581 CrossRef CAS; (e) T. Zweifel, M. Nielsen, J. Overgaard, C. B. Jacobsen and K. A. Jørgensen, Eur. J. Org. Chem., 2011, 1, 47 CrossRef; (f) T. Inokuchi and H. Kawafuchi, J. Org. Chem., 2006, 71, 947 CrossRef CAS; (g) C. Xing, X. Li, S. Zhu, J. Zhao and S. Zhu, Tetrahedron Lett., 2006, 47, 4951 CrossRef CAS; (h) J. Yang, H. Li, M. Li, J. Peng and Y. Gu, Adv. Synth. Catal., 2012, 354, 688 CrossRef CAS; (i) D. Enders, A. Grossmann, H. Huang and G. Raabe, Eur. J. Org. Chem., 2011, 4298 CrossRef CAS; (j) C. Borsch, K. L. Jensen, J. Udmark and K. A. Jørgensen, Org. Lett., 2011, 13, 4790 CrossRef; (k) N. Samakkanad, P. Katrun, T. Techajaroonjit, S. Hlekhlai, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram and C. Kuhakarn, Synthesis, 2012, 44, 1693 CrossRef CAS.
- J. Peña, B. A. Ana, R. F. Moro, I. S. Marcos, N. M. Garrido and D. Díez, Tetrahedron, 2011, 67, 8331 CrossRef.
- (a) J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2012, 29, 144 RSC and references cited therein; (b) I. S. Marcos, L. Castañeda, P. Basabe, D. Díez and J. G. Urones, Mini-Rev. Org. Chem., 2012, 9, 54 CrossRef CAS; (c) J. G. Urones, J. De Pascual Teresa, I. S. Marcos, D. Díez, N. M. Garrido and R. A. Guerra, Phytochemistry, 1987, 26, 1077 CrossRef CAS; (d) R. M. Young, W. L. Popplewell, M. R. Caira, R. L. van Zyl and M. T. Davies-Coleman, J. Chem. Res., 2011, 18 CrossRef CAS; (e) A. W. W. van Wyk and M. T. Davies-Coleman, Tetrahedron, 2007, 63, 12179 CrossRef CAS; (f) R. J. Peters, M. M. Ravn, R. M. Coates and R. B. Croteau, J. Am. Chem. Soc., 2001, 123, 8974 CrossRef CAS; (g) See also: A. Shigeru, Aromatopia, 2009, 94, 10 Search PubMed.
- B. List, R. A. Lerner and C. F. Barbas III, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS.
- (a) B. Alcaide, P. Almendros, A. Luna and M. R. Torres, J. Org. Chem., 2006, 71, 4818 CrossRef CAS; (b) J. T. Suri, S. Mitsumori, K. Albertshofer, F. Tanka and C. F. Barbas III, J. Org. Chem., 2006, 71, 3822 CrossRef CAS; (c) C. Grondal and D. Enders, Tetrahedron, 2006, 62, 329 CrossRef CAS; (d) J. T. Suri, D. B. Ramachary and C. F. Barbas III, Org. Lett., 2005, 7, 1383 CrossRef CAS; (e) I. Ibrahem and A. Córdova, Tetrahedron Lett., 2005, 46, 3363 CrossRef CAS; (f) R. I. Storer and D. W. C. MacMillan, Tetrahedron, 2004, 60, 7705 CrossRef CAS; (g) J. Casas, H. Sundén and A. Córdova, Tetrahedron Lett., 2004, 45, 6117 CrossRef CAS; (h) Q. Pan, B. Zou, Y. Wang and D. Ma, Org. Lett., 2004, 6, 1009 CrossRef CAS; (i) A. B. Northrup, I. K. Mangion, F. Hettche and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2004, 43, 2152 CrossRef CAS; (j) C. Aleman, R. Gordillo, F. R. Clemente, P. H.-Y. Cheong and K. N. Houk, Acc. Chem. Res., 2004, 37, 558 CrossRef.
- (a) J. M. Janey, Y. Hsiao and J. D. Armstrong III., J. Org. Chem., 2006, 71, 390 CrossRef CAS; (b) A. Córdova, W. Notz, G. Zhong, J. M. Betancort and C. F. Barbas III, J. Am. Chem. Soc., 2002, 124, 1842 CrossRef; (c) B. List, P. Pojarliev, W. T. Biller and H. J. Martin, J. Am. Chem. Soc., 2002, 124, 827 CrossRef CAS; (d) W. Notz, F. Tanaka, S. Watanabe, N. S. Chowdari, J. M. Turner, R. Thayumanavan and C. F. Barbas III, J. Org. Chem., 2003, 68, 9624 CrossRef CAS; (e) N. S. Chowdari, J. T. Suri and C. F. Barbas III, Org. Lett., 2004, 6, 2507 CrossRef CAS; (f) Y. Hayashi, W. Tsuboi, I. Ashimine, T. Urushima, M. Shoji and K. Sakai, Angew. Chem., Int. Ed., 2003, 42, 3677 CrossRef CAS.
- (a) S. Hanessian and V. Pham, Org. Lett., 2000, 2, 2975 CrossRef CAS; (b) B. List, P. Pojarliev and H. J. Martin, Org. Lett., 2001, 3, 2423 CrossRef CAS; (c) J. M. Betancort and C. F. Barbas III, Org. Lett., 2001, 3, 3737 CrossRef CAS; (d) I. K. Mangion and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 3696 CrossRef CAS.
- (a) J. S. Yadav, S. P. Kumar, G. Kondaji, R. S. Rao and K. Nagaiah, Chem. Lett., 2004, 33, 1168 CrossRef CAS; (b) J. Mabry and B. Ganem, Tetrahedron Lett., 2006, 47, 55 CrossRef CAS.
- D. B. Ramachary, N. S. Chowdari and C. F. Barbas III, Angew. Chem., Int. Ed., 2003, 42, 4233 CrossRef CAS.
- (a) M. Shi, J. K. Jiang and C. Q. Li, Tetrahedron Lett., 2002, 43, 127 CrossRef CAS; (b) S. H. Chen, B. C. Hong, C. F. Su and S. Sarshar, Tetrahedron Lett., 2005, 46, 8899 CrossRef CAS; (c) J. E. Imbriglio, M. M. Vasbinder and S. J. Miller, Org. Lett., 2003, 5, 3741 CrossRef CAS.
- (a) N. Utsumi, H. Zhang, F. Tanaka and C. F. Barbas III, Angew. Chem., Int. Ed., 2007, 46, 1878 CrossRef CAS; (b) J. Vesely, P. Dziedzic and A. Córdova, Tetrahedron Lett., 2007, 48, 6900 CrossRef CAS.
- J. Wang, H. Li, Y. Mei, B. Lou, D. Xu, D. Xie, H. Guo and W. Wang, J. Org. Chem., 2005, 70, 5678 CrossRef CAS.
- M. P. Brochu, S. P.Brown and D. W. C. MacMillan, J. Am. Chem. Soc., 2004, 126, 4108 CrossRef CAS.
- (a) G. Zhong, Angew. Chem., Int. Ed., 2003, 42, 4247 CrossRef CAS; (b) S. P. Brown, M. P. Brochu, C. J. Sinz and D. W. C MacMillan, J. Am. Chem. Soc., 2003, 125, 10808 CrossRef CAS; (c) Y. Hayashi, J. Yamaguchi, K. Hibino and M. Shoji, Tetrahedron Lett., 2003, 44, 8293 CrossRef CAS; (d) A. Bøgevig, H. Sundén and A Córdova, Angew. Chem., Int. Ed., 2004, 43, 1109 CrossRef; (e) Y. Hayashi, J. Yamaguchi, K. Hibino and M. Shoji, Angew. Chem., Int. Ed., 2004, 43, 1112 CrossRef CAS; (f) Y. Hayashi, J. Yamaguchi, T. Sumiya, K. Hibino and M. Shoji, J. Org. Chem., 2004, 69, 5966 CrossRef CAS; (g) A. Córdova, H. Sundén, A. Bøgevig, M. Johansson and F. Himo, Chem.–Eur. J., 2004, 10, 3673 CrossRef.
- (a) S. M. Rajesh, B. D. Bala, S. Perumal and J. C. Menéndez, Green Chem., 2011, 13, 3248 RSC; (b) A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 14, 290 RSC; (c) M. Srinivasan and S. Perumal, Tetrahedron, 2007, 63, 2865 CrossRef CAS; (d) S. Indumathi, S. Perumal and J. C. Menéndez, J. Org. Chem., 2010, 75, 472 CrossRef CAS; (e) S. Indumathi, S. Perumal and J. C. Menéndez, Tetrahedron, 2011, 67, 7101 CrossRef CAS; (f) J. Pandey, N. Anand and R. P. Tripathi, Tetrahedron, 2009, 65, 9350 CrossRef CAS; (g) A. Kumar, M. Kumar and M. K. Gupta, Tetrahedron Lett., 2009, 50, 7024 CrossRef CAS; (h) A. Kumar and R. A. Maurya, Tetrahedron, 2008, 64, 3477 CrossRef CAS; (i) A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946 CrossRef CAS; (j) H. Jiang, R. Mai, H. Cao, Q. Zhu and X. Liu, Org. Biomol. Chem., 2009, 7, 4943 RSC; (k) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (l) R. C. Wende and P. R. Schreiner, Green Chem., 2012, 14, 1821 RSC.
- (a) For recent reviews on organocatalysis e.g. see: Proc. Natl. Acad. Sci., USA, 2010, 107, 48 Search PubMed(special feature issue on organocatalysis); (b) Chem. Rev., 2007, 107, 12, 5413–5883 Search PubMed (rev. special issue on organocatalysis); (c) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS; (d) M. J. Gaunt, C. C. C. Johansson, A. McNally and N. C. Vo, Drug Discovery Today, 2007, 2, 8 CrossRef; (e) P. I. Dalko, Enantioselective Organocatalysis, Wiley-VCH, Weinheim, 2007 Search PubMed; (f) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC; (g) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4703 CrossRef CAS . See also; (h) S. K. Panday, Tetrahedron: Asymmetry, 2011, 22, 1817 CrossRef CAS; (i) S. Abele, R. Inauen, D. Spielvogel and C. Moessner, J. Org. Chem., 2012, 77, 4765 CrossRef CAS; (j) E. Marqués-López, R. P. Herrera and M. Christmann, Nat. Prod. Rep., 2010, 27, 1138 RSC.
- See the complete Issue 4 of Chem. Soc. Rev., 2012, 41 Search PubMed.
- For the reaction of a β-oxosulfone with an enal e.g. see: (a) Ref. 7a ; (b) C. Xing, X. Li, S. Zhu, J. Zhao and S. Zhu, Tetrahedron Lett., 2006, 47, 4951 CrossRef CAS.
- (a) For some uses of chiral phosphoric acids in the synthesis of 2H-pyrans e.g. see: J. Moreau, C. Hubert, J. Batany, L. Toupet, T. Roisnel, J-P. Hurvois and J-L. Renaud, J. Org. Chem., 2009, 74, 8963 CrossRef CAS; (b) B. Guo, G. Schwarzwalder and J. T. Njardarson, Angew. Chem., Int. Ed., 2012, 51, 1 CrossRef; (c) H. Zhao, Y-B. Lan, Z-M. Liu, Y. Wang, X-W Wang and J-C. Tao, Eur. J. Org. Chem., 2012, 1935 CrossRef CAS.
- B. J. Bench, C. Liu, C. R. Evett and C. M. H. Watanabe, J. Org. Chem., 2006, 71, 9458 CrossRef CAS.
- For an excellent highlight in enantioselective 6π-electrocyclizations see: J. L Vicario and D. Badia, Chem. Cat. Chem., 2010, 2, 375 CAS.
- M. J. Riveira and M. P. Mischne, Chem.–Eur. J., 2012, 18, 2382 CrossRef CAS.
- G. Appendino, G. Cravotto, S. Tagliapietra, G. M. Nano and G. Palmisano, Helv. Chim. Acta, 1990, 73, 1805 CrossRef.
- (a) C. J. O'Connor, H. S. G. Beckmann and R. S. David, Chem. Soc. Rev., 2012, 41, 4444 RSC; (b) M. A. Burke and S. L. Screiber, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef; (c) S. Screiber, Science, 2000, 287, 1964 CrossRef; (d) See also: H. R. Safaei, M. Shekouhy, S. Rahmanpur and A. Shirinfeshan, Green Chem., 2012, 14, 1696 RSC.
- K. F. Bernady, M. B. Floyd, J. F. Poletto and M. J. Weiss, J. Org. Chem., 1979, 44, 1438 CrossRef CAS.
- S. Xie, S. Uesato, T. Fujitaand and H. Inouye, J. Nat. Prod., 1989, 52, 701 CrossRef CAS.
- K. Ishihara, H. Ishibashi and H. Yamamoto, J. Am. Chem. Soc., 2002, 124, 3647 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: General information, experimental details and copies of representative spectra. See DOI: 10.1039/c2ra21306k/ |
| This journal is © The Royal Society of Chemistry 2012 |

