Observation of flower-like patterns in syndiotactic polystyrene/carbon nanotube nanocomposite films†
Jinhao
Zhang
ab,
Jianjun
Wang
a,
Guangming
Chen
*a,
Cuiping
Yuan
ab and
Jiping
Yang
*b
aBeijing National Laboratory for Molecular Sciences (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: chengm@iccas.ac.cn.
bSchool of Materials Science and Engineering, Beihang University, Beijing, 100191, P. R. China. E-mail: jyang@buaa.edu.cn
First published on 10th July 2012
Abstract
Unusual flower-like patterns were observed for the first time in syndiotactic polystyrene/carbon nanotube nanocomposite films, which were prepared by simple hot-pressing using polyimide films or aluminium foils as supporting substrates.
In recent years, due to the unique structural and excellent mechanical, conductive and thermal properties of carbon nanotubes (CNTs),1,2 polymer/CNT nanocomposites have attracted extensive interest from the viewpoints of both fundamental science and technical applications.3–6 However, most of this research has concentrated on nanocomposite preparation, CNT dispersion and alignment, the effect of CNTs on polymer crystallization, and the enhanced properties for the polymer/CNT nanocomposites.3–6 Studies of patterned structures in polymer/CNT nanocomposite films are scarce according to our literature survey.
It is well known that polymer thin films are normally unstable structures, being kinetically frozen and far from equilibrium. Recently, some patterns in polymer thin films due to dewetting or phase-separation or phase-inversion have garnered great interest in the fields of bio-related and medicinal industries, optoelectronics, semiconductors, and coatings.7–11 Nevertheless, these reported patterns are relatively simple in morphology. Simple structures such as featureless granules or droplets and stripe-like networks are the commonly encountered patterns.9–17 Complex patterns, which should have promising applications, have seldom been reported in polymer films to date. Indeed, to our knowledge, complex flower-like patterns in polymer based nanocomposite films have not been reported so far.
Very recently, we prepared syndiotactic polystyrene/multi-walled CNT (sPS/MWCNT) nanocomposites by simple solution mixing.18 Their crystallization,18 orientation19,20 and other properties18 were also studied. Herein, we report an observation of unusual flower-like patterns in the hot-pressed sPS/MWCNT nanocomposite films. The nanocomposite films, with thickness of about 80 μm, were obtained by a simple hot-pressing procedure under 10 MPa for 10 min (ESI, preparation of sPS/MWCNT nanocomposite films†).
Fig. 1 shows a typical photographic picture of the hot-pressed thin film for the sPS/MWCNT nanocomposite using polyimide film substrates. The yellow color background results from the polyimide film at the bottom, while the black color originates from the MWCNTs in the nanocomposite. In Fig. 1, many flower-like patterns with high transparency are clearly observed. The sizes of most patterns have a range of 0.6–2.2 mm in diameter. More interestingly, the morphology of the blooming-flower-like patterns is versatile. For example, arrow a points to the mono-flower-like pattern, wherein the pattern is composed of only one flower. Some patterns are composed of two (arrow b) or three (arrow c) flowers combined together in one pattern, i.e. two-in-one or three-in-one flower-like patterns. In addition, flower-cluster-like patterns made up of many flowers can be observed (arrow d). Besides the above blooming-flower-like patterns, there are some embryotic-bud-like patterns (arrow e). Therefore, Fig. 1 reveals enriched information on complex flower-like patterns with high transparency, which include embryotic-bud-like and full-blooming-flower-like patterns, ranging from mono-flower-like to flower-cluster-like patterns. These observed flower-like patterns are unusual, and have not been reported so far. Alternatively, we note that the preparation method is very convenient, using only simple hot-pressing to afford the polymer/CNT nanocomposite films.
 | ||
| Fig. 1 Image of flower-like patterns for the sPS/MWCNT nanocomposite (1 wt.% MWCNTs) film with polyimide film at the bottom, prepared by hot-pressing the nanocomposite between two polyimide film substrates at 300 °C under 10 MPa for 10 min. The arrows (a–e) refer to (a) mono-flower-like, (b) two-in-one flower-like, (c) three-in-one flower-like, (d) flower-cluster-like, and (e) embryotic-bud-like patterns. | ||
Generally, the substrate material is believed to play an important role in the formation of film patterns. In order to investigate the effect of the substrate material on film patterns, aluminium foil was employed. Fig. 2 displays a typical photographic image of the hot-pressed sPS/WMCNT nanocomposite film using aluminium foil as a substrate. When compared with Fig. 1, the shape of the flower-like patterns in Fig. 2 are essentially similar. However, the sizes of the flower-like patterns are apparently larger than those displayed in Fig. 1. For instance, most of the mono-flower-like patterns in Fig. 2 are ca. 1.0–5.2 mm in size, while Fig. 1 displays mono-flower-like patterns of about 0.6–2.2 mm. Alternatively, aside from the various patterns shown in Fig. 1, shish-kebab-like patterns can be clearly observed in Fig. 2, as pointed out by the arrows. Small flower-like or bud-like patterns (kebabs) appear along a straight shish. Obviously, when compared with the relatively simple stripes or droplet-like patterns prepared by conventional dewetting, phase-separation or phase-inversion,9–17 the flower-like patterns reported herein are more complex and informative, which may attract intense and extensive interest from both fundamental and technological viewpoints.
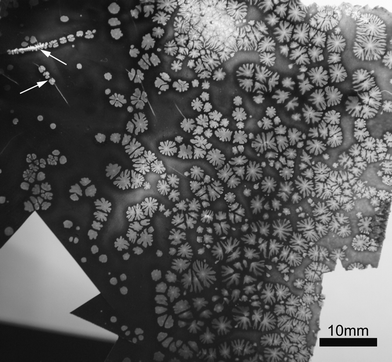 | ||
| Fig. 2 Image of flower-like patterns for the sPS/MWCNT nanocomposite (1 wt% MWCNTs) film on aluminium foil, prepared by hot-pressing between two aluminium foil substrates at 300 °C under 10 MPa for 10 min. The arrows point to the shish-kebab-like patterns. Scale bar is 10 mm. | ||
To investigate the internal structure of the flower-like patterns, the nanocomposite films were fractured, and the sectioned surface was measured by SEM. Fig. 3A clearly demonstrates that the transparent flower-like patterns are made up of cavities sandwiched by two thin boundary layers with thickness around 9 μm. More detailed studies shown in Figs. 3B and C further elucidate that the MWCNT distribution is quite different between the bulk (B) and the boundary (C). The bulk has a much higher content of MWCNTs than the boundary layer. Furthermore, the obtained sPS/MWCNT nanocomposite film containing the flower-like patterns was etched in a mixture of KMnO4/H2SO4/H3PO4 (0.60 g, 20 mL, and 10 mL, respectively). Then, a top-view of the etched film was observed. In Fig. 4A, an image of a mono-flower-like pattern is displayed. The flower-like pattern has a diameter of around 1.8 mm. It is evident that the internal structure of the millimeter-scale flower-like pattern is a cavity, which is consistent with the above results of Fig. 3. Fig. 4B shows an enlargement of the interface region of the etched flower-like pattern. Interestingly, the MWCNTs become enriched in the interface region, which is possibly due to the gaseous solvent evaporation and the resultant pushing effect during the hot-pressing procedure. Additionally, Raman spectroscopic measurements were conducted to study the difference of the MWCNT distribution. The normalized Raman spectroscopic results are shown in Fig. 5, wherein the band at 3056 cm−1, characteristic of the C–H stretching of phenyl rings for the sPS, is chosen as the reference band. The intensities of all the Raman spectroscopic bands derived from the MWCNTs, including D (1332 cm−1), G (1584 cm−1) and G' (2662 cm−1) bands, for the bulk are much higher than those of the corresponding bands for the boundary layer of flower-like patterns, which strongly supports the SEM results shown in Figs. 3 and 4. Therefore, the results of both SEM and Raman spectra demonstrate that the transparent flower-like patterns are formed by cavities sandwiched by two adjacent boundary layers with dramatically reduced MWCNT content.
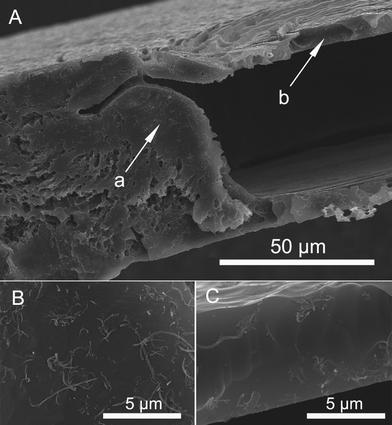 | ||
| Fig. 3 SEM images of sectioned planes for the sPS/MWCNT nanocomposite film. B (bulk) and C (boundary layer) are the enlargment of areas pointed by arrows a and b in A, respectively. | ||
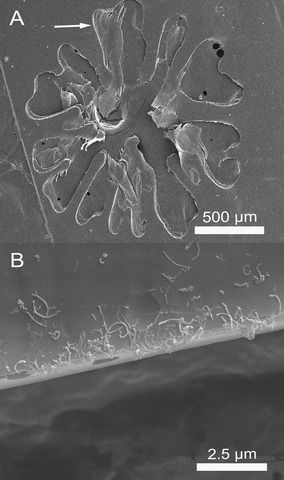 | ||
| Fig. 4 SEM images of the top-view of the etched product for the hot-pressed sPS/MWCNT nanocomposite film. (A) is an etched flower-like pattern, and (B) is the enlargement of the interface region, pointed by an arrow in (A). | ||
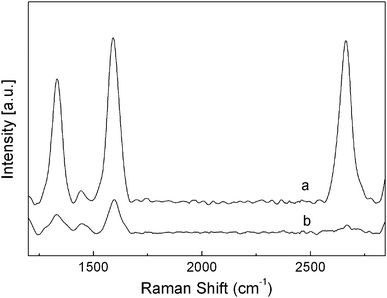 | ||
| Fig. 5 Normalized Raman spectra of the bulk (a) and boundary layer of the flower-like pattern (b) for the sPS/MWCNT nanocomposite film. The band at 3056 cm−1 is chosen as the reference band. | ||
In order to elucidate the formation mechanism of the flower-like patterns, the corresponding simply-melt-mixed product of the sPS/MWNCT composite was used for comparison, and the effect of quenching as well as sPS crystallization were taken into consideration. Fig. S1 (ESI†) shows that no flower-like patterns occur at all. Only large and black agglomerates of the MWCNTs exist due to drastic MWCNT aggregation. In sharp contrast, by solution dispersion, using N-methyl-2-pyrrolidinone (NMP) as the medium, the MWCNTs could be separated and homogeneously dispersed in the sPS matrix for the nanocomposite.18 Therefore, we believe that the MWCNT homogeneous dispersion and the remaining NMP solvent may play important roles in the formation of the flower-like patterns.
In addition, Fig. S2 (ESI†) presents the XRD patterns of the hot-pressed nanocomposite films after being slowly cooled down (A) and rapidly quenched (B), respectively. Obviously, the nanocomposite film in Fig. S2A (ESI†) exhibits a similar crystalline phase for both the regions of the bulk (a) and the flower-like patterns (b). SPS is a semicrystalline polymer with complex crystalline phase and phase transformation.21–25 The sharp peaks at 2θ = 6.2°, 10.4°, 12.4°, 13.6°, 18.7°, 20.2°, and 21.4° correspond to the β-form crystals—the thermodynamically stable crystalline phase.22–25 Moreover, the degree of crystallinity for the boundary layer of the flower-like pattern (a) seems obviously higher than that in the bulk (b). As evidenced by Figs. 3 and 4, the boundary layer of the flowerlike pattern has a relatively low content of MWCNTs. This may account for its high crystallinity due to the reduced interference of MWCNTs in the sPS crystallization, which agrees with our previous investigations18 very well. As for the quenched film (Fig. S2B, ESI†), only two broad and featureless peaks at ca. 10 and 20°, characteristic of the amorphous phase of the sPS,15,25 appear. Both films clearly show similar flower-like patterns, suggesting that crystallization of the sPS has little effect on the flower-like pattern formation. Therefore, we conclude that the MWCNT homogeneous dispersion and the remained NMP molecules are required for the formation of the flower-like patterns, whereas quenching and sPS crystallization are not.
Additionally, the main reason for the deviation of pattern size between Figs. 1 and 2 and the occurrence of shish-kebab-like patterns in Fig. 2 may lie in the difference between the thermal conductivity and the wrinkling of the substrate materials. On the one hand, aluminum has a much higher thermal conductivity than polyimide. The velocity of heat transfer for aluminum is much faster, and the thermal distribution is much more uniform. These factors might contribute greatly to the rapidly growth of the cavities, once the cavities appear inside the nanocomposite films under hot-pressing. As a consequence, the flower-like patterns using aluminum foil substrates are obviously larger than those with polyimide film substrates. On the other hand, because aluminum foil is easy to wrinkle, shish-kebab-like patterns might occur when flower-like or bud-like patterns form along the folding (Fig. 2). In contrast, polyimide films do not wrinkle easily. As a result, no such shish-kebab-like patterns take place in the sPS/MWCNT nanocomposite films being hot-pressed between polyimide films (Fig. 1).
In summary, we report herein an observation of unusual flower-like patterns in polymer/CNT nanocomposite films prepared by simple hot-pressing. Versatile transparent flower-like patterns including mono-flower-like, two-in-one flower-like, three-in-one flower-like and flower-cluster-like as well as embryotic-bud-like patterns were clearly observed in the hot-pressed sPS/MWCNT nanocomposite films. Due to the wrinkling of aluminium foils, some shish-kebab-like patterns exist when using aluminium foil as a substrate. Additionally, the sizes of the patterns using aluminium foil substrates are larger than those prepared using polyimide film substrates. Further structural investigations reveal that the transparent flower-like patterns are composed of cavities sandwiched by two transparent boundary layers with dramatically reduced content of the MWCNTs, and the MWCNTs become enriched in the interface region. We believe that the homogeneous dispersion of MWCNTs and the remaining organic solvents are required for the formation of the complex flower-like patterns, whereas quenching and sPS crystallization have little effect.
This work is financially support by the National Natural Science Foundation of China (50873103). G. Chen acknowledges the support of the K. C. Wong Education Foundation, Hong Kong.
References
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- M. F. Yu, O. Lourie, M. J. Dyer, K. M. Monoli, T. F. Kelly and R. S. Ruoff, Science, 2000, 287, 637 CrossRef CAS.
- B. Vigolo, A. Penicaud, C. Coulon, C. Sauder, R. Pailler, C. Journet, P. Bernier and P. Poulin, Science, 2010, 290, 1331 CrossRef.
- L. Yang, F. Liu, H. Xia, X. Qian, K. Shen and J. Zhang, Carbon, 2011, 49, 3274 CrossRef CAS.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194 CrossRef CAS.
- G. Sun, G. Chen, J. Liu, J. Yang, J. Xie, Z. Liu, R. Li and X. Li, Polymer, 2009, 50, 5787 CrossRef CAS.
- E. Menard, M. A. Meitl, Y. Sun, J. Park, D. J. Shir, Y. Nam, S. Jeon and J. A. Rogers, Chem. Rev., 2007, 107, 1117 CrossRef CAS.
- S. Liu, W. Wang, A. L. Briseno, S. C. B. Mannsfeld and Z. Bao, Adv. Mater., 2009, 21, 1217 CrossRef CAS.
- L. Xue and Y. Han, Prog. Polym. Sci., 2011, 36, 269 CrossRef CAS.
- J. Ye and C. V. Thompson, Adv. Mater., 2011, 23, 1567 CrossRef CAS.
- M. Ramanathan and S. B. Darling, Prog. Polym. Sci., 2011, 36, 793 CrossRef CAS.
- A. Verma and A. Sharma, Adv. Mater., 2010, 22, 5306 CrossRef CAS.
- R. A. Farrell, N. Kehagias, M. T. Shaw, V. Reboud, M. Zelsmann, J. D. Holmes, C. M. S. Torres and M. A. Morris, ACS Nano, 2011, 5, 1073 CrossRef CAS.
- H. C. Shum, E. Santanach-Carreras, J.-W. Kim, A. Ehrlicher, J. Bibette and D. A. Weitz, J. Am. Chem. Soc., 2011, 133, 4420 CrossRef CAS.
- K. A. Barnes, J. F. Douglas, D.-W. Liu and A. Karim, Adv. Colloid Interface Sci., 2001, 94, 83 CrossRef CAS.
- K. A. Barnes, A. Karim, J. F. Douglas, A. I. Nakatani, H. Gruell and E. J. Amis, Macromolecules, 2000, 33, 477 CrossRef.
- Y.-S. Sun, S.-W. Chien, J.-Y. Liou, C. H. Su and K.-F. Liao, Polymer, 2011, 52, 1180 CrossRef CAS.
- G. Sun, G. Chen, Z. Liu and M. Chen, Carbon, 2010, 48, 1434 CrossRef CAS.
- C. Yuan, J. Zhang, G. Chen and J. Yang, Chem. Commun., 2011, 47, 899 RSC.
- C. Yuan, J. Wang, G. Chen, J. Zhang and J. Yang, Soft Matter, 2011, 7, 4039 RSC.
- E. B. Gowd, K. Tashiro and C. Ramesh, Prog. Polym. Sci., 2009, 34, 280 CrossRef CAS.
- T. Ouchi, S. Nagasaka and A. Hotta, Macromolecules, 2011, 44, 2112 CrossRef CAS.
- A. K. Dikshit and A. Kaito, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 1665 CrossRef CAS.
- C. Wang, C.-L. Huang, Y.-C. Chen, G.-L. Hwang and S.-J. Tsai, Polymer, 2008, 49, 5564 CrossRef CAS.
- Y. Sun and E. M. Woo, Polymer, 2001, 42, 2241 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and preparation of the sPS/MWCNT nanocomposite films, digital image, and XRD patterns. See DOI: 10.1039/c2ra21090h/ |
| This journal is © The Royal Society of Chemistry 2012 |
