Recyclable Pd–graphene catalyst: mechanistic insights into heterogeneous and homogeneous catalysis†
Yuta
Nishina
*a,
Junya
Miyata
a,
Ryo
Kawai
b and
Kazuma
Gotoh
b
aResearch Core for Interdisciplinary Sciences, Okayama University, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan. E-mail: nisina-y@cc.okayama-u.ac.jp; Fax: (+81) 86-251-8718; Tel: (+81) 86-251-8718
bGraduate School of Natural Science and Technology, Okayama University, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan
First published on 23rd August 2012
Abstract
Pd–graphene, a composite of Pd nanoparticles and exfoliated graphene, was used as a catalyst in organic reactions. In the Suzuki–Miyaura cross-coupling reaction, Pd–graphene showed high reusability without aggregation of Pd nanoparticles. The intermediates of the reaction in the liquid phase, Ph–Pd–Br and Ph–Pd–OH, were detected by ESI-MS.
Graphene is a two-dimensional sheet of sp2-hybridized carbon. Because of its extraordinary thermal, mechanical, and electrical properties, graphene has attracted much interest both in theoretical studies and applications.1 One possible route to improving these properties for applications would be to use graphene in composite materials,2 especially as a support for metal nanoparticles.3 Metal–graphene composite materials have been used as catalysts for fuel cells,4 sensors,5 and solar cells.6 Although metal–graphene composite materials have high surface areas and stabilities, only a few studies have involved their application as heterogeneous catalysts in organic reactions.7 In the field of catalysis, Pd is particularly important among the noble metals.8 Pd-supported inorganic materials are known as active catalysts.9 In addition, immobilization or stabilization of Pd nanoparticles in organic matrixes have also been developed.10 Pd supported on graphite oxide (GtO) has recently been reported to act as a catalyst in the Suzuki–Miyaura cross-coupling reaction.11
We have developed a new technique for preparing metal–graphene composite materials via intercalation of metal cations into graphene oxide (GO) layers followed by thermal exfoliation.12 The Pd–graphene composite has a high BET specific surface area of over 500 m2 g−1. TEM observations (Fig. 1a) indicate a good dispersion of Pd nanoparticles (average diameter 2–5 nm) and the carbon thin films supporting the Pd nanoparticles consist of mono- and few-layered graphene sheets. TG mass loss analysis revealed that 10.6 wt% of Pd is deposited onto the graphene sheet (see ESI†).
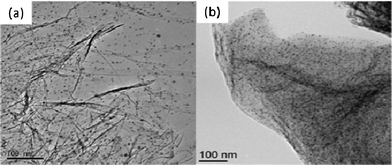 | ||
| Fig. 1 Micrographs of Pd–graphene (1): (a) TEM image of 1 before the reaction and (b) TEM image of 1 recovered after the 2nd cycle and dried in vacuo. | ||
Initially, we investigated the Suzuki–Miyaura coupling reaction, a well-known reaction catalysed by Pd, which is a powerful and convenient synthetic method in organic chemistry for generating biaryls, conducting polymers, and liquid crystals.13 The catalytic effectiveness of different Pd–graphene-type composite materials was investigated (Table 1). A Pd–graphene catalyst (1), prepared using an exfoliation method,12 gave the product in quantitative yield (entry 1). In contrast, Pd–GtO (2), which has a layered structure prepared using a previously reported method,11a gave a low yield (entry 2). Pd–GO (3), a precursor of Pd–graphene (1) containing mainly Pd(II) species, did not show high catalytic activity under the present reaction conditions (entry 3).
|
|
||
|---|---|---|
| Entry | Catalyst | Yield/% |
| a Bromobenzene (0.20 mmol), phenylboronic acid (0.22 mmol), K2CO3 (0.3 mmol), catalyst (1 mg), EtOH (0.25 mL), H2O (0.25 mL). | ||
| 1 | Pd–graphene 1 | quant. |
| 2 | Pd–GO 2 | 35 |
| 3 | Pd–GO 3 | 13 |
Previously reported heterogeneous Pd catalysts underwent aggregation of Pd nanoparticles, causing a decrease in product yield when reused as a result of a decrease in the Pd surface area.11a The Pd–graphene catalyst (1) was found to be recyclable; however, the yield of the product decreased after the third cycle (Table 2, cycle 4). TEM measurement of catalyst 1 revealed that the Pd particle size did not change before and after the reaction (Fig. 1). This indicates that the catalyst deactivation is not caused by aggregation of Pd. The recovered Pd–graphene catalyst was calcined in air at 300 °C for 30 min, washed with H2O and EtOH, and dried in vacuo to remove impurities adsorbed onto the catalyst. After these catalyst re-activation procedures, the catalytic ability of the Pd–graphene was restored (Table 2, cycle 6).
|
|
||||||
|---|---|---|---|---|---|---|
| a Bromobenzene (0.20 mmol), phenylboronic acid (0.22 mmol), K2CO3 (0.3 mmol), catalyst (1 mg), EtOH (0.25 mL), H2O (0.25 mL). b The catalyst was calcined at 300 °C for 1 h, washed, and dried, then reused. | ||||||
| Cycle | 1 | 2 | 3 | 4 | 5 | 6b |
| Yield/% | quant. | 97 | 98 | 58 | 34 | 88 |
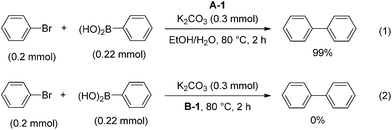
It is necessary to determine whether the reaction occurs on the surface of the Pd particles or with leached homogeneous Pd species. First, Pd–graphene (1) and K2CO3 were stirred in EtOH and H2O at 80 °C for 2 h, followed by filtration to obtain a solid material (A-1) and solution (B-1) (Scheme 1). Each of these was used for a Suzuki–Miyaura coupling reaction. When a catalytic amount of A-1 was used in the reaction of bromobenzene and phenylboronic acid, biphenyl was obtained quantitatively (eqn (1)). In contrast, when B-1 was used as the solvent in the reaction, without a catalyst, no reaction occurred (eqn (2)). These results indicate that leaching of Pd did not occur as a result of heating Pd–graphene (1).
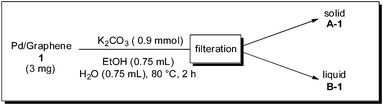 | ||
| Scheme 1 Leaching experiment 1. | ||
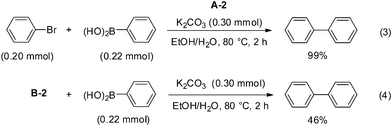
Next, Pd–graphene (1) and bromobenzene were heated in EtOH and H2O at 80 °C for 2 h, followed by filtration, to obtain a solid material (A-2) and solution (B-2) (Scheme 2). Each of these was used for the reaction. As in the case of the A-1-catalyzed reaction (eqn (1)), A-2 gave the product quantitatively (eqn (3)). Interestingly, when B-2 was used as the solvent, the product was obtained in 46% yield (eqn (4)). This result suggests that Pd(II) species formed by oxidative addition of Pd(0) to bromobenzene were leached into the solvent. We tried to confirm the formation of Ph–Pd–Br species using ESI-MS.14 Pd–graphene (1) (5 mg, Pd content: 0.009 mmol) and bromobenzene (2 mg, 0.013 mmol) were heated in EtOH and H2O for 30 min. After filtration, the solution was analyzed by ESI(+)-MS using MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the eluent. We observed peaks with m/z = 201 and 265, corresponding to Ph–Pd–OH and Ph–Pd–Br species, respectively (Fig. 2). This suggests that the Pd was leached by forming Pd(II) with bromobenzene.
1 as the eluent. We observed peaks with m/z = 201 and 265, corresponding to Ph–Pd–OH and Ph–Pd–Br species, respectively (Fig. 2). This suggests that the Pd was leached by forming Pd(II) with bromobenzene.
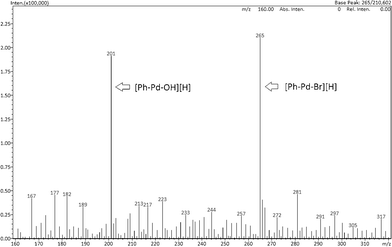 | ||
| Fig. 2 ESI(+)-MS spectra of Pd–graphene and bromobenzene mixture after heating at 80 °C for 30 min in EtOH and H2O. | ||
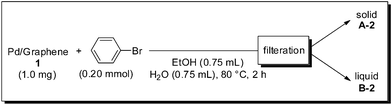 | ||
| Scheme 2 Leaching experiment 2. | ||
Although we have shown that homogeneous Pd species could act as an active catalyst, the amount of leached Pd would be small, because the Pd particle size was the same before and after the reaction (Fig. 1). We performed ICP-MS analysis of the filtered crude reaction mixture; 406 ppb of Pd were observed in the liquid phase. It is known that the leaching of Pd from common heterogeneous Pd catalysts ranges from several parts per million to more than 100 ppm.15 If only 406 ppb (1.9 × 10−6 mmol) of Pd could catalyse the reaction shown in eqn (4), the turnover number (TON) of Pd would be 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. This value approximately corresponds to that of a homogeneous palladacycle catalyst.16 The TON increased to 300
000. This value approximately corresponds to that of a homogeneous palladacycle catalyst.16 The TON increased to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 when the reaction was performed on a 50 mmol scale for 24 h. In an actual reaction system, Pd would be partially released from and captured on the graphene surface. After the reductive elimination, the formation of Pd(0) causes the redeposition of Pd on the support. Graphene's sheet structure would prevent aggregation. Other supports such as activated carbon have high surface area, however, the pore size of activated carbon would be too small for Pd(0) to disperse, causing aggregation. This point needs further experiments and discussions to clarify the reason.
000 when the reaction was performed on a 50 mmol scale for 24 h. In an actual reaction system, Pd would be partially released from and captured on the graphene surface. After the reductive elimination, the formation of Pd(0) causes the redeposition of Pd on the support. Graphene's sheet structure would prevent aggregation. Other supports such as activated carbon have high surface area, however, the pore size of activated carbon would be too small for Pd(0) to disperse, causing aggregation. This point needs further experiments and discussions to clarify the reason.
Pd–graphene (1) showed catalytic activity not only in cross-coupling reactions but also in hydrogenation of unsaturated molecules, and could be recycled at least three times (eqn (5))17 and selective hydrothiolation of alkynes (eqn (6)).18 These results indicate that Pd–graphene has both heterogeneous and homogeneous Pd catalyst characteristics.
In conclusion, Pd–graphene prepared by the original technique17 worked as an efficient catalyst in organic reactions. The mechanistic investigation suggested the formation of homogeneous Pd(II) species in the actual reaction system. The operational simplicity, relatively low catalyst loading, excellent recyclability, and low catalyst leaching make this catalyst more attractive for future application.
Acknowledgements
This study was financially supported by the Funds for the Development of Human Resources in Science and Technology and RCIS-Young Faculty Joint Research Award.References
- (a) J. C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, T. J. Booth and S. Roth, Nature, 2007, 446, 60–63 CrossRef CAS; (b) L. A. Ponomarenko, F. Schedin, M. I. Katsnelson, R. Yang, E. W. Hill, K. S. Novoselov and A. K. Geim, Science, 2008, 320, 356–358 CrossRef CAS; (c) K. S. Novoselov, Z. Jiang, Y. Zhang, S. V. Morozov, H. L. Stormer, U. Zeitler, J. C. Maan, G. S. Boebinger, P. Kim and A. K. Geim, Science, 2007, 315, 1379–1379 CrossRef CAS; (d) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS.
- (a) S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS; (b) T. Ramanathan, A. A. Abdala, S. Stankovich, D. A. Dikin, M. Herrera-Alonso, R. D. Piner, D. H. Adamson, H. C. Schniepp, X. Chen, R. S. Ruoff, S. T. Nguyen, I. A. Aksay, R. K. Prud'homme and L. C. Brinson, Nat. Nanotechnol., 2008, 3, 327–331 CrossRef CAS.
- (a) R. Muszynski, B. Seger and P. V. Kamat, J. Phys. Chem. C, 2008, 112, 5263–5266 CrossRef CAS; (b) X. Z. Zhou, X. Huang, X. Y. Qi, S. X. Wu, C. Xue, F. Y. C. Boey, Q. Y. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CrossRef CAS; (c) H. M. A. Hassan, V. Abdelsayed, A. E. R. S. Khder, K. M. AbouZeid, J. Terner, M. S. El-Shall, S. I. Al-Resayes and A. A. El-Azhary, J. Mater. Chem., 2009, 19, 3832–3837 RSC.
- (a) E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura and I. Honma, Nano Lett., 2009, 9, 2255–2259 CrossRef CAS; (b) Y. J. Li, W. Gao, L. J. Ci, C. M. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124–1130 CrossRef CAS; (c) H. J. Huang, H. Q. Chen, D. P. Sun and X. Wang, J. Power Sources, 2012, 204, 46–52 CrossRef CAS.
- (a) S. Liu, J. Q. Tian, L. Wang and X. P. Sun, J. Nanopart. Res., 2011, 13, 4539–4548 CrossRef CAS; (b) J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Adv. Mater., 2010, 22, 4877–4880 CrossRef CAS.
- (a) X. Wang, L. J. Zhi and K. Mullen, Nano Lett., 2008, 8, 323–327 CrossRef CAS; (b) S. R. Sun, L. Gao and Y. Q. Liu, Appl. Phys. Lett., 2010, 96, 083113 CrossRef.
- (a) A. Mastalir, T. Szabo, Z. Kiraly and I. Dekany, Catal. Commun., 2012, 17, 104–107 CrossRef CAS; (b) Y. Li, X. B. Fan, J. J. Qi, J. Y. Ji, S. L. Wang, G. L. Zhang and F. B. Zhang, Nano Res., 2010, 3, 429–437 CrossRef CAS; (c) Y. Li, X. B. Fan, J. J. Qi, J. Y. Ji, S. L. Wang, G. L. Zhang and F. B. Zhang, Mater. Res. Bull., 2010, 45, 1413–1418 CrossRef CAS; (d) G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mulhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS; (e) J. Bian, M. Xiao, S. J. Wang, Y. X. Lu and Y. Z. Meng, Catal. Commun., 2009, 10, 1529–1533 CrossRef CAS; (f) A. Mastalir, Z. Kiraly, M. Benko and I. Dekany, Catal. Lett., 2008, 124, 34–38 CrossRef CAS.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) H. U. Blaser, A. Indolese, A. Schnyder, H. Steiner and M. Studer, J. Mol. Catal. A: Chem., 2001, 173, 3–18 CrossRef CAS; (c) N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef CAS; (d) L. X. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS; (e) L. Q. Xue and Z. Y. Lin, Chem. Soc. Rev., 2010, 39, 1692–1705 RSC.
- (a) Recent reports on catalytic reactions using Pd-inorganic composites, T. Maegawa, Y. Kitamura, S. Sako, T. Udzu, A. Sakurai, A. Tanaka, Y. Kobayashi, K. Endo, U. Bora, T. Kurita, A. Kozaki, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2007, 13, 5937–5943 CrossRef CAS; (b) J. S. Huang, D. W. Wang, H. Q. Hou and T. Y. You, Adv. Funct. Mater., 2008, 18, 441–448 CrossRef CAS; (c) R. B. Bedford, U. G. Singh, R. I. Walton, R. T. Williams and S. A. Davis, Chem. Mater., 2005, 17, 701–707 CrossRef CAS; (d) N. Erathodiyil, S. Ooi, A. M. Seayad, Y. Han, S. S. Lee and J. Y. Ying, Chem.–Eur. J., 2008, 14, 3118–3125 CrossRef CAS; (e) A. Gniewek, J. J. Ziolkowski, A. M. Trzeciak, M. Zawadzki, H. Grabowska and J. Wrzyszcz, J. Catal., 2008, 254, 121–130 CrossRef CAS; (f) L. M. Neal and H. E. Hagelin-Weaver, J. Mol. Catal. A: Chem., 2008, 284, 141–148 CrossRef CAS; (g) F. Li, Q. H. Zhang and Y. Wang, Appl. Catal., A, 2008, 334, 217–226 CrossRef CAS.
- (a) Recent reports on catalytic reactions using Pd-organic composites, X. Yang, Z. F. Fei, D. B. Zhao, W. H. Ang, Y. D. Li and P. J. Dyson, Inorg. Chem., 2008, 47, 3292–3297 CrossRef CAS; (b) J. D. Scholten, B. C. Leal and J. Dupont, ACS Catal., 2012, 2, 184–200 CrossRef CAS; (c) R. Touzani and H. Alper, J. Mol. Catal. A: Chem., 2005, 227, 197–207 CrossRef CAS; (d) D. Astruc, C. Ornelas, A. K. Diallo and J. Ruiz, Molecules, 2010, 15, 4947–4960 CrossRef CAS; (e) R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340–8347 CrossRef CAS; (f) B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251–7254 CrossRef CAS.
- (a) G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mulhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS; (b) S. Moussa, A. R. Siamaki, B. F. Gupton and M. S. El-Shall, ACS Catal., 2012, 2, 145–154 CrossRef CAS; (c) A. R. Siamaki, A. E. S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catal., 2011, 279, 1–11 CrossRef CAS.
- K. Gotoh, T. Kinumoto, E. Fujii, A. Yamamoto, H. Hashimoto, T. Ohkubo, A. Itadani, Y. Kuroda and H. Ishida, Carbon, 2011, 49, 1118–1125 CrossRef CAS.
- (a) A. Suzuki, J. Organomet. Chem., 1999, 576, 147–168 CrossRef CAS; (b) J. Hassan, E. Schulz, C. Gozzi and M. Lemaire, J. Mol. Catal. A: Chem., 2003, 195, 125–131 CrossRef CAS; (c) M. Hird, G. W. Gray and K. J. Toyne, Mol. Cryst. Liq. Cryst., 1991, 206, 187–204 CrossRef CAS; (d) J. A. McCubbin, X. Tong, R. Y. Wang, Y. Zhao, V. Snieckus and R. P. Lemieux, J. Am. Chem. Soc., 2004, 126, 1161–1167 CrossRef CAS.
- (a) C. Raminelli, M. H. G. Prechtl, L. S. Santos, M. N. Eberlin and J. V. Comasseto, Organometallics, 2004, 23, 3990–3996 CrossRef CAS; (b) C. Chevrin, J. Le Bras, A. Roglans, D. Harakat and J. Muzart, New J. Chem., 2007, 31, 121–126 RSC; (c) J. Wassenaar, E. Jansen, W. J. van Zeist, F. M. Bickelhaupt, M. A. Siegler, A. L. Spek and J. N. H. Reek, Nat. Chem., 2010, 2, 417–421 CrossRef CAS.
- (a) I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139–10140 CrossRef CAS; (b) F. Y. Zhao, M. Shirai, Y. Ikushima and M. Arai, J. Mol. Catal. A: Chem., 2002, 180, 211–219 CrossRef CAS.
- M. Beller, H. Fischer, W. A. Herrmann, K. Ofele and C. Brossmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 1848–1849 CrossRef CAS.
- (a) Selected examples of palladium-catalyzed hydrogenation of alkynes. D. Teschner, E. Vass, M. Havecker, S. Zafeiratos, P. Schnorch, H. Sauer, A. Knop-Gericke, R. Schloegl, M. Chamam, A. Wootsch, A. S. Canning, J. J. Gamman, S. D. Jackson, J. McGregor and L. F. Gladden, J. Catal., 2006, 242, 26–37 CrossRef CAS; (b) L. Guczi, Z. Schay, G. Stefler, L. F. Liotta, G. Deganello and A. M. Venezia, J. Catal., 1999, 182, 456–462 CrossRef CAS.
- (a) Selected examples of palladium-catalyzed hydrothiolation of alkynes. A. Ogawa, T. Ikeda, K. Kimura and T. Hirao, J. Am. Chem. Soc., 1999, 121, 5108–5114 CrossRef CAS; (b) H. Kuniyasu, A. Ogawa, K. I. Sato, I. Ryu, N. Kambe and N. Sonoda, J. Am. Chem. Soc., 1992, 114, 5902–5903 CrossRef CAS; (c) C. J. Weiss, S. D. Wobser and T. J. Marks, Organometallics, 2010, 29, 6308–6320 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, characterization data. See DOI: 10.1039/c2ra21185h |
| This journal is © The Royal Society of Chemistry 2012 |