Microstructural investigation of concentric-circled poly(L-lactic acid) spherulites with self-assembled nanofibrils
Wei-Chi
Lai
*ab
aDepartment of Chemical and Materials Engineering, Tamkang University, No.151, Yingzhuan Rd., Tamsui Dist., New Taipei City, 25137, Taiwan. E-mail: wclai@mail.tku.edu.tw; Fax: +886-2-2620-9887; Tel: +886-2-2621-5656 ext.3516
bEnergy and Opto-Electronic Materials Research Center, Tamkang University, No.151, Yingzhuan Rd., Tamsui Dist., New Taipei City, 25137, Taiwan
First published on 13th June 2012
Abstract
The morphology and microstructure of poly(L-lactic acid) (PLLA) with different amounts of self-assembled 1,3:2,4-dibenzylidene-D-sorbitol (DBS) nanofibrils were examined using polarizing optical microscopy (POM), scanning electron microscopy (SEM), and small-angle X-ray scattering (SAXS). A small amount of DBS (less than 4 wt% DBS) changed the spherulitic morphologies and microstructures of PLLA. The formation of concentric-circled PLLA spherulites was due to the aggregation of DBS nanofibrils around the inside circles (or inside the spherulites). The concentric-circled spherulites were easily obtained at high DBS contents and high PLLA crystallization temperatures. However, at higher crystallization temperatures, the average diameter of the inside circles would exceed the final spherulite size of PLLA. Meanwhile, the DBS nanofibrils were mostly outside the spherulites. Therefore, the concentric-circled structures were not observed. Both the DBS content and PLLA crystallization temperature influenced the formation of concentric-circled spherulites. Moreover, PLLA crystallization temperatures seemed to have slightly larger effects. The crystallization temperature affected the nucleation and spherulite growth rate of PLLA, and thus altered the aggregation of DBS molecules and nanofibrils thereof. SAXS results indicated the long period of the PLLA samples was not significantly influenced by the formation of the concentric-circled spherulites. However, the maximum peak of PLLA broadened when DBS was added. The DBS nanofibrils influenced the regularity of PLLA lamellae, leading to the broader peak.
Introduction
DBS is a crystalline solid, with a melting point around 225 °C.1 It is an amphiphilic molecule derived from the natural sugar alcohol D-glucitol. The chemical structure is given in ref. 1. It can self-assemble into a fibrillar network by hydrogen bonds at low concentrations in some organic solvents to produce organogels.2–9 The resulting nanofibrils range from 10 nm to 0.8 μm in diameter, as measured by electron microscopy. POM observations show that these organogels exhibit a spherulitic-like morphology.10 Neat DBS also revealed a spherulite-like morphology during cooling from the melt.10 The diameters of the aggregated DBS fibrils ranged from 100 nm to 1 μm, which were larger than those in the DBS organogel systems. Neat DBS is usually used as the nucleating agent to change the crystallization rates and optical properties of semicrystalline polymers such as poly(ethylene terephthalate) (PET), polyethylene (PE), and polypropylene (PP).11–13Poly (L-lactic acid) (PLLA) is a biodegradable semicrystalline polymer. Much attention has been paid to it due to its commercial applications as an environmentally friendly and biomedical material. It is well known that the properties of a polymer can be well controlled by the morphology. The morphology of a polymer, especially for a semicrystalline polymer, plays an important role in determining its physical and mechanical properties. For a biodegradable PLLA system, many investigators have reported the effect of the morphology on the thermal properties, hydrolytic degradation, and film porosity of PLLA.14–16 Therefore, we believe the structure and morphology of PLLA is very important and deserving of research. Moreover, the different spherulitic morphologies of PLLA have been the subject of much research in the field of polymer physics in the last decade.17–24 The PLLA morphology conventionally exhibits Maltese crosses with a birefringent pattern (typical spherulite). However, other structures, such as ring-banded spherulites, were also found in some PLLA systems.17–24 In earlier research, the ring-banded spherulites, which were not observed for neat PLLA, were identified for blends or copolymers. Many researchers suggested that the ringed pattern is associated with the spatial twisting of lamellae.17–24 For example, poly (D,L-lactide) (PDLLA) in the PLLA/PDLLA blend system altered the aggregation and twisting of PLLA lamellae, and led to the appearance of such a pattern.21 In recent years, banded structures was also observed in neat PLLA through different annealing procedures or slow cooling rates.22–24
In our previous study,25 intriguing new structures of PLLA in the presence of DBS were observed. We term these new structures “concentric-circled spherulites”. They are unlike typical PLLA spherulites or ringed-banded spherulites, which display periodic extinction rings. The circles appeared only once. The formation of concentric-circled spherulites was due to the aggregation of DBS fibrils around the circles, but some dispersed nanofibrils still formed beyond the circles. These dispersed nanofibrils affected the orientation of the PLLA lamellae and caused a change in the birefringence. In this study, we observed the microstructure and morphology of the PLLA concentric-circled spherulites formed in the presence of DBS in detail. The transitions of spherulitic morphologies of PLLA were also investigated. The main factors influencing the change of the typical spherulites into concentric-circled spherulites of PLLA were discussed as well. In addition, small-angle X-ray scattering (SAXS) was used to clarify the microstructures of these samples.
Furthermore, we measured the diameter of the circles and the average time required to form them within the concentric-circled spherulites, and discussed these in relation to the composition and crystallization temperature, as in a system having ring-banded spherulites.26,27 The influencing factors on the formation of concentric-circled PLLA spherulites were then analyzed.
Experimental
Materials
Poly(L-lactic acid) (PLLA) was purchased from NatureWorks Company. 1,3:2,4-dibenzylidene-D-sorbitol (DBS) was obtained from Milliken Chemicals Company.Sample preparation
Preparation of DBS/PLLA samples was done using the solution-casting method. They were dissolved in chloroform yielding a 2% w/v solution. The solution was then poured onto a glass dish. A film was obtained after the solvent was evaporated slowly at room temperature under ambient conditions. The film was then dried in a vacuum at 80 °C for 24 h. TGA was used to check the residual solvent in the final films. The results showed no residual solvent in the final films. The DBS contents in the PLLA samples were up to 5 wt%. Beyond that percentage, DBS did not dissolve completely, and the solution became opaque. As a result, DBS concentrations from 0 to 4 wt% in the PLLA samples were chosen for the following experiments.Spherulitic morphology, POM
The spherulitic morphology of the DBS/PLLA samples was monitored with an Olympus CX41 polarizing optical microscope (POM). The samples were placed on glass, heated to 240 °C, and held for 1 min on a Linkam THMS600 hot stage. The samples were then quickly cooled to their crystallization temperature, where the resultant morphology was observed.Microstructure, SEM
The microstructures of the DBS/PLLA samples were observed using a Leo-1530 field emission scanning electron microscopy (FE-SEM). The POM samples were used in the SEM experiments.Microstructure, SAXS
The microstructure of the PLLA samples with 0–4 wt% DBS was conducted using a Bruker Nanostar Small-Angle X-ray Scattering (SAXS) instrument. The X-ray source, a 1.5 KW X-ray generator (Kristalloflex 760) equipped with a rotating anode Cu target, was operated at 35 mA and 40 KV. The scattering intensities were detected by a 2-D position-sensitive detector (Bruker AXS). The sample-detector distance was 65 cm, and the X-ray wavelength was 0.154 nm. The intensity profiles were output as the plots of the scattering intensity (I) versus the scattering vector, q = 4π/λsin(θ/2) (θ = scattering angle). The samples were placed on glasses, heated to 240 °C, and held for 1 min on a Linkam THMS600 hot stage. Then, the samples were cooled to their crystallization temperature (120 °C). Following the crystallization, the samples were removed from the glass and probed by SAXS at room temperature.Results and discussion
SEM and POM
DBS is an amphiphilic molecule that can self-organize into fibrils from the melt during cooling. In our previous study,25 we found that DBS molecules still self-assemble into nanofibrils during the PLLA crystallization process, although there are some intermolecular interactions, such as hydrogen bonding, between PLLA and DBS. The new PLLA structures “concentric-circled spherulites” are found in DBS/PLLA systems. Fig. 1 shows the POM and SEM micrographs of the concentric-circled spherulites in PLLA samples with 4 wt% DBS crystallized at 120 °C. One of the concentric circles is indicated by the arrow. It is clear that the concentric circles appeared as brighter parts in the SEM micrograph. The upper right corner in Fig. 1(b) displays an enlarged magnification of the brighter area. Significant aggregation of DBS nanofibrils can be observed. Due to the phase separation between crystalline PLLA and DBS, the DBS concentration outside the spherulites increased as the PLLA crystallized. When the concentrations of aggregated DBS molecules were high enough, DBS nanofibrils formed. If DBS molecules were less aggregated, no fibril structure formed.25 As seen in Fig. 1, most of the nanofibrils aggregated around the “concentric circles” (or the “inside circles”), and the diameters of these nanofibrils were around 10–20 nm. Some nanofibrils formed beyond the circles and are dispersed in the spherulites. These dispersed DBS nanofibrils caused a difference in the birefringence of the sample.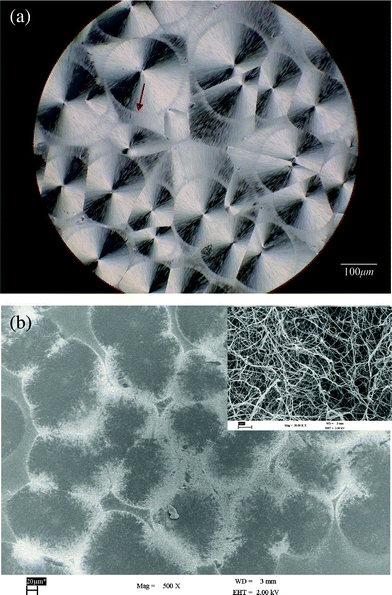 | ||
| Fig. 1 (a) POM photograph of the concentric-circled spherulites (one of which is indicated by the arrow) in PLLA samples with 4 wt% DBS crystallized at 120 °C, and (b) SEM micrograph with the upper right corner displaying an enlarged magnification of the brighter area. | ||
Fig. 2 plots the distribution of the spherulitic morphologies of the DBS/PLLA samples at different PLLA crystallization temperatures and DBS amounts, as observed by POM. The formation of PLLA spherulites was not suppressed during cooling as the crystallization temperature was less than 100 °C. Therefore, the experiment for the crystallization temperature was chosen between 100 °C and 140 °C. Note that no concentric-circled spherulites were observed in samples with DBS contents below 2 wt%. As seen in Fig. 2, samples under these conditions displayed two different PLLA spherulitic morphologies. For samples crystallized at temperature below 120 °C, the typical spherulites changed into the concentric-circled spherulites at higher DBS amounts and crystallization temperatures. The morphology of the spherulites is related to the DBS amount and PLLA crystallization temperature. However, when the samples were crystallized at higher crystallization temperatures (above 120 °C), the concentric-circled spherulites disappeared and typical spherulite structures appeared again. Moreover, the concentric-circled structures disappeared more easily at lower DBS amounts and higher crystallization temperatures. The reasons for these will be explained later.
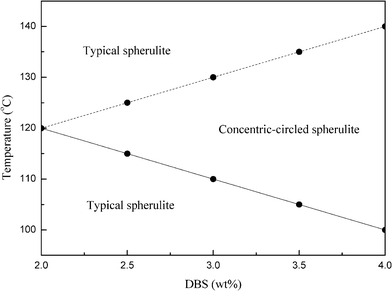 | ||
| Fig. 2 Distribution of the spherulitic morphologies of the DBS/PLLA samples at different PLLA crystallization temperatures and DBS amounts, as observed by POM. | ||
Two characteristics of the concentric-circled spherulites were further analyzed: the average diameter of the inside circles (ρ) and the average time required to form them (t) within the concentric-circled spherulites. Fig. 3 displays ρ and t as a function of DBS content for PLLA samples with 3–4 wt% DBS. It was found ρ (Fig. 3 (a)) and t (Fig. 3 (b)) both decreased with an increase in DBS content for all samples. That is, the concentric-circled PLLA spherulites formed more easily as the DBS content was increased. This could be explained by the larger aggregation of DBS molecules, which led to the earlier formation of DBS nanofibrils. Fig. 4 plots ρ and t as a function of PLLA crystallization temperature for PLLA samples with 3–4 wt% DBS. ρ (Fig. 4 (a)) and t (Fig. 4 (b)) increased when the crystallization temperature of PLLA increased. The increase in ρ and t indicates it needed more time to form the DBS nanofibrils. The inset in Fig. 4(a) displays the spherulitic growth rate of the neat PLLA as a function of the crystallization temperature. The growth rate was measured from the linear regression of the spherulite radius as a function of time, as observed by POM. The reason for the increase in ρ and t could be the growth rate of PLLA. The spherulitic growth rate of PLLA increased in the range 100–130 °C. The higher growth rate of PLLA means the amorphous components (including melted DBS) could not be excluded easily, and more time was required for the aggregated DBS molecules to reach a concentration high enough to form the nanofibrils. In addition, ρ and t should decrease due to the decreased spherulitic growth rate for the samples crystallized at 140 °C (see the inset in Fig. 4(a)). However, the tendency was the opposite. Moreover, the concentric-circled structures were observed only in the samples containing 4 wt% DBS. We believe nucleation could be the reason. At a high crystallization temperature, it was difficult to form stable PLLA nuclei, and there were fewer PLLA nuclei. The small number of PLLA nuclei caused minimal aggregation of DBS molecules, and therefore the formation of the nanofibrils was difficult at such a low DBS concentration. Higher DBS amounts might offset this effect, so the concentric-circled spherulites were still observed in the samples with 4 wt% DBS. In brief, the influence of the crystallization temperature on the formation of the concentric-circled PLLA spherulites includes not only the spherulite growth rate but also the nucleation effect, especially at higher temperatures.
 | ||
| Fig. 3 (a) ρ and (b) t as a function of DBS content for PLLA samples with 3–4 wt% DBS. | ||
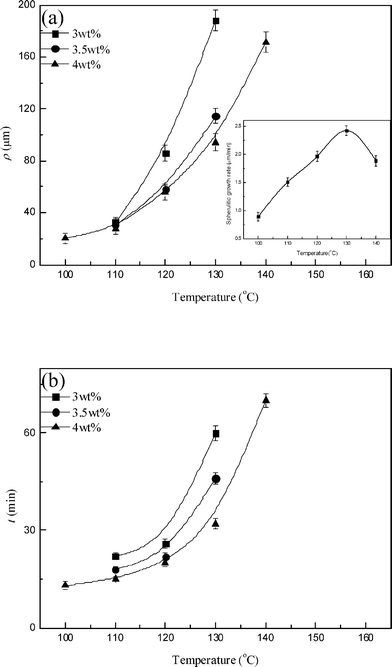 | ||
| Fig. 4 (a) ρ and (b) t as a function of PLLA crystallization temperature for PLLA samples with 3–4 wt% DBS. The inset in Fig. 4(a) displays the spherulitic growth rate of the neat PLLA as a function of the crystallization temperature. | ||
Fig. 5 shows ρ and the final spherulite size (effective diameter) as a function of PLLA crystallization temperature for the samples with 3–4 wt% DBS. For the sample containing 3 wt% DBS, ρ was close to the final spherulite size of PLLA at a crystallization temperature of 130 °C (see Fig. 5(a)). At higher crystallization temperatures, the concentric-circled structures disappeared (see Fig. 2) though the relationship between ρ and the crystallization temperature suggests that larger inside circles should be observed. Similarly, the ρ values were close to the final spherulite sizes of PLLA at crystallization temperatures of 135 and 140 °C, respectively, for the samples with 3.5 and 4 wt% DBS (see Fig. 5(b),(c)), and the concentric-circled structures disappeared beyond those temperatures (see Fig. 2). It appears that the concentric-circled spherulites disappeared because ρ would exceed the final spherulite size of PLLA at higher crystallization temperatures.
 | ||
| Fig. 5 ρ and final spherulite size as a function of PLLA crystallization temperature for the samples with (a) 3, (b) 3.5 and (c) 4 wt% DBS. | ||
Fig. 6 displays the SEM photographs of the PLLA sample containing 3.5 wt% DBS crystallized at 140 °C. Although no concentric-circled structures were observed, DBS nanofibrils were still present. As can be seen, some parts of the image, in particular the interstices and spherulitic boundaries, were brighter than others. The enlarged SEM photographs (Fig. 6(b),(c)) clearly show that DBS nanofibrils with diameters of around 10–20 nm aggregated outside the PLLA spherulites. This could explain why no concentric-circled spherulites were observed at higher crystallization temperatures. The formation of concentric-circled PLLA spherulites was due to the earlier existence of DBS nanofibrils aggregating inside the spherulites (see Fig. 1) during the PLLA crystallization process. As shown in Fig. 5, ρ increased with the crystallization temperature. Above certain temperatures, depending on the DBS content, ρ would be expected to be larger than the final spherulite size of PLLA. Hence, DBS nanofibrils should aggregate outside the spherulites. Since the spherulites were virtually in contact with one another, DBS nanofibrils were mostly at the interstices and boundaries. Note that the amount of DBS nanofibrils in Fig. 6 was obviously less than that in Fig. 1, although the DBS content was higher. This is probably because of the nucleation effect, which, as mentioned above, should be considered at high temperatures. Less PLLA nucleation led to less aggregation of DBS molecules and thus smaller numbers of the nanofibrils.
 | ||
Fig. 6 SEM photographs ((a) 500 X, (b) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 X, and (c) 50 000 X, and (c) 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 X) of the PLLA sample containing 3.5 wt% DBS crystallized at 140 °C. 000 X) of the PLLA sample containing 3.5 wt% DBS crystallized at 140 °C. | ||
The concentric-circled structures were more difficult to form with decreasing DBS amount and increasing PLLA crystallization temperature. As Fig. 3(b) and Fig. 4 (b) show, more time was needed to form the nanofibrils (to form the concentric-circled structures) for the samples with lower amounts of DBS crystallized at higher temperatures. Moreover, as seen in Fig. 3(a) and Fig. 4 (a), ρ was also found to increase with a decrease in DBS amount and an increase in PLLA crystallization temperature. Larger ρ indicates greater possibility of being larger than the final spherulite size of PLLA. Therefore, this caused the concentric-circled structures to disappear much more easily.
Fig. 7(a) and (b) show the relationship between the ρ and t of samples with different DBS amounts crystallized at various PLLA crystallization temperatures. It was found that both factors (DBS contents and PLLA crystallization temperatures) influenced the formation of concentric-circled spherulites. Moreover, the inconsistent trend suggests that the effect of PLLA crystallization temperature was slightly more prominent. The crystallization temperature affected the growth rate and nucleation of PLLA, thus altering the aggregation of DBS molecules and nanofibrils, and influencing the formation of concentric-circled spherulites.
 | ||
| Fig. 7 Relationship between ρ and t of samples with different DBS amounts crystallized at various PLLA crystallization temperatures. Data points are grouped according to (a) DBS amount and (b) crystallization temperature, respectively. | ||
SAXS
SAXS was used to elucidate the microstructures of the PLLA samples. Fig. 8 displays the SAXS spectra, I(q) vs. q and I(q)q2vs. q (Lorentz-corrected), of the PLLA samples with different amount of DBS crystallized at 120 °C. No peaks were found in the neat DBS sample in the q-range (< 1.0 nm−1). Kawai et al.28 and Nam et al.29 found that the primary, maximum peak position (qm) in the lamellar level, such as the long period of PLLA, was around 0.3–0.4 nm−1. As can be seen in Fig. 8(a), qm (around 0.35 nm−1, indicated by the arrows) was not significantly changed by the addition of DBS. The respective Bragg d spacings at these qm values dspacing = 2π/qm) were around 18 nm. When the DBS amount was greater than 2 wt%, the typical spherulites of PLLA changed into concentric-circled spherulites at the crystallization temperature of 120 °C. Therefore, the formation of the concentric-circled spherulites does not seem to influence the long period of PLLA significantly. However, the maximum peak broadened when DBS was added (see Fig. 8(b), where the range of the peak is indicated by the arrows). The noise was removed for easier comparison. The half-widths at half-maximum of the maximum peak were 0.041, 0.043, 0.072 and 0.077 for the samples with increasing DBS contents. A possible explanation is that the DBS nanofibrils influenced the regularity of PLLA lamellae, leading to the broader peak.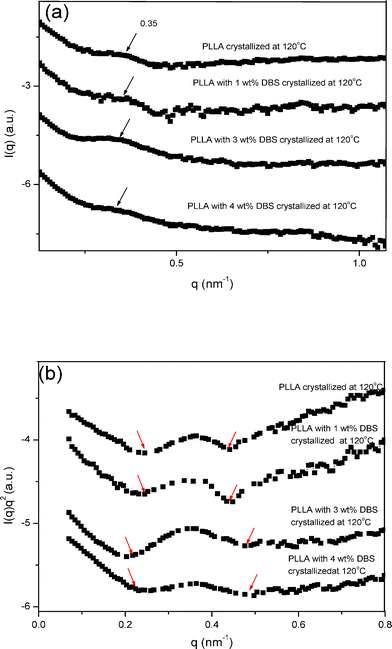 | ||
| Fig. 8 SAXS spectra, I(q) vs. q and I(q)q2vs. q (Lorentz-corrected), of the PLLA samples with different amount of DBS crystallized at 120 °C. | ||
Conclusions
The morphology and microstructure of PLLA with DBS was observed. The typical spherulites of PLLA changed into concentric-circled spherulites of PLLA at high PLLA crystallization temperatures and high amounts of DBS. The average diameter of the inside circles (ρ) and the average time required to form them (t) for all samples decreased as the DBS amount increased. This means the concentric-circled PLLA spherulites formed easily when the DBS contents were increased. However, ρ and t increased when the crystallization temperature of PLLA increased. The increased spherulitic growth rate of PLLA made the amorphous components (including melted DBS) more difficult to exclude. Therefore, it needed much time to be excluded outside the crystals. Moreover, at high PLLA crystallization temperatures, the nucleation effect should be considered for the formation of the concentric-circled PLLA spherulites. PLLA had difficulty forming stable nuclei at high crystallization temperatures, and the number of nuclei of PLLA was less. The small number of PLLA nuclei caused the small aggregation of DBS molecules, and the DBS nanofibrils were thus hard to form. Moreover, at higher crystallization temperatures (above 120 °C), the concentric-circled spherulites disappeared and typical spherulite structures reappeared. The concentric-circled spherulites disappeared because ρ would exceed the final spherulite size of PLLA at higher crystallization temperatures. In addition, ρ increased with decreasing amounts of DBS and increasing crystallization temperatures. A larger ρ was more easily larger than the final spherulite size of PLLA. Therefore, the concentric-circled structures were more difficult to form. Furthermore, the formation of the PLLA concentric-circled spherulites was affected by both the PLLA crystallization temperature and DBS amount, yet the former seemed to have slightly larger effects. The PLLA crystallization temperature affected the growth rate and nucleation of PLLA, thus influencing the formation of concentric-circled spherulites. The SAXS study showed the long period of PLLA was not influenced significantly by the formation of the concentric-circled spherulites. However, the DBS nanofibrils may have affected the regularity of PLLA lamellae, leading to a broader peak.Acknowledgements
We gratefully acknowledge financial support from the Taiwan National Science Council (NSC 99-2221-E-032-006). We also thank Mr. Po-Yu Chen and Mr. Jia-Pei Liao for assisting with some experiments.References
- T. Bauer, R. Thomann and R. Mulhaupt, Macromolecules, 1998, 31, 7651–7658 CrossRef CAS.
- E. A. Wilder, C. K. Hall, S. A. Khan and R. J. Spontak, Langmuir, 2003, 19, 6004–6013 CrossRef CAS.
- E. A. Wilder, C. K. Hall and R. J. Spontak, J. Colloid Interface Sci., 2003, 267, 509–518 CrossRef CAS.
- E. A. Wilder, M. Braunfeld, B. H. Jinnai, C. K. Hall, D. A. Agard and R. J. Spontak, J. Phys. Chem. B, 2003, 107, 11633–11642 CrossRef CAS.
- J. Lipp, M. Shuster, A. E. Terry and Y. Cohen, Langmuir, 2006, 22, 6398–6402 CrossRef CAS.
- W. C. Lai, S. C. Tseng, S. H Tung, Y. E. Huang and S. R. Raghavan, J. Phys. Chem. B, 2009, 113, 8026–8030 CrossRef CAS.
- D. J. Mercurio and R. J. Spontak, J. Phys. Chem. B, 2001, 105, 2091–2098 CrossRef CAS.
- W. C. Lai, S. C. Tseng and Y. S. Chao, Langmuir, 2011, 27, 12630–12635 CrossRef CAS.
- W. Chen, Y. Yang, C. H. Lee and A. Q. Shen, Langmuir, 2008, 24, 10432–10436 CrossRef CAS.
- W. C. Lai and C. H. Wu, J. Appl. Polym. Sci., 2010, 115, 1113–1119 CrossRef CAS.
- J. Cao, K. Wang, W. Cao, Q. Zhang, R. Du and Q. Fu, J. Appl. Polym. Sci., 2009, 112, 1104–1113 CrossRef CAS.
- M. Kristiansen, M. Werner, T. Tervoort and P. Smith, Macromolecules, 2003, 36, 5150–5156 CrossRef CAS.
- K. Wang, C. J. Zhou, C. Y. Tang, Q. Zhang, R. N. Du, Q. Fu and L. Li, Polymer, 2009, 50, 696–706 CrossRef CAS.
- H. Pan and Z. Qiu, Macromolecules, 2010, 43, 1499–1506 CrossRef CAS.
- H. Sawalha, K. Schroën and R. Boom, J. Appl. Polym. Sci., 2008, 107, 82–93 CrossRef CAS.
- F. Teraoka, M. Hara, M. Nakagawa and T. Sohmura, J. Appl. Polym. Sci., 2010, 117, 1566–1571 CAS.
- J. Li, Y. Li, J. Zhou, J. Yang, Z. Jiang, P. Chen, Y. Wang, Q. Gu and Z. Wang, Macromolecules, 2011, 44, 2918–2925 CrossRef CAS.
- Y. Li, X. Liu, D. Chao, L. Cui and W. Zhang, Polym. J., 2008, 40, 1195–1198 CrossRef CAS.
- J. Xu, B. H. Guo, J. J. Zhou, L. Li, J. Wu and M. Kowalczuk, Polymer, 2005, 46, 9176–9185 CrossRef CAS.
- I. Ohkoshi, H. Abe and Y. Doi, Polymer, 2000, 41, 5985–5992 CrossRef CAS.
- P. Pan, Z. Liang, B. Zhu, T. Dong and Y. Inoue, Macromolecules, 2009, 42, 3374–3380 CrossRef CAS.
- Y. Wang and J. F. Mano, J. Appl. Polym. Sci., 2007, 105, 3500–3504 CrossRef CAS.
- S. Nurkhamidah and E. M. Woo, J. Appl. Polym. Sci., 2011, 122, 1976–1985 CrossRef CAS.
- M. L. Di Lorenzo, Eur. Polym. J., 2005, 41, 569–575 CrossRef CAS.
- W. C. Lai, Soft Matter, 2011, 7, 3844–3851 RSC.
- S. Nojima, K. Watanabe, Z. Y. Zheng and T. Ashida, Polym. J., 1988, 20, 823–826 CrossRef CAS.
- Z. Wang, X. Wang, D. Yu and B. Jiang, Polymer, 1997, 38, 5897–5901 CrossRef CAS.
- T. Kawai, N. Rahman, G. Matsuba, K. Nishida, T. Kanaya, M. Nakano, H. Okamoto, J. Kawada, A. Usuki, N. Honma, K. Nakajima and M. Matsuda, Macromolecules, 2007, 40, 9463–9469 CrossRef CAS.
- P. H. Nam, N. Ninomiya, A. Fujimori and T. Masuko, Polym. Eng. Sci., 2006, 46, 703–711 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
