Synthesis, characterization, photolytic degradation, electrical conductivity and applications of a nanocomposite adsorbent for the treatment of pollutants
Mohammad
Shahadat
*a,
S. A.
Nabi
a,
Rani
Bushra
a,
A. S.
Raeissi
a,
K.
Umar
a and
Mohd Omaish
Ansari
b
aAnalytical Research Laboratory, Department of Chemistry, Aligarh Muslim University, Aligarh 202002, India. E-mail: mdshahadat93@gmail.com; Tel: +91-571-2404014
bDepartment of Applied Chemistry, Aligarh Muslim University, Aligarh 202002, India
First published on 10th July 2012
Abstract
A new semi-crystalline nanocomposite cation exchanger has been synthesized by the sol–gel method and characterized on the basis of FTIR, XRD, SEM, TEM, TGA and CHNO analysis. The ion-exchange material was synthesized at pH 1.0 and shows an ion exchange capacity of 1.37 meq g−1 for Na+ ions. The composite material exhibits improved ion exchange capacity along with chemical and thermal stability. It can be used at up to 300 °C, with 98% retention of its initial ion-exchange capacity. The conductivity of different samples of composite material was found to be in the semiconducting range, which indicates that the conductivity of the samples is highly dependent on the % of inorganic precipitate. The photochemical degradation of industrial dye was also investigated using this composite. The distribution coefficient studies of metal ions on the material were performed in different concentrations of surfactants (Tween 80, CPC) and on mixtures (solvent + surfactant). On the basis of Kd values the material was found to be selective for Hg(II), Bi(III), Zr(IV) and Pb(II) ions. Some analytically important separations of metal ions in synthetic mixtures and real samples were achieved on the column of this exchanger. The limits of detection for Pb(II), Zn(II) and Hg(II) were found to be 0.32, 0.92 and 0.50 μg L−1 and the limits of quantification were found to be 1.07, 3.08 and 1.69, respectively. Besides the ion-exchanger, polyaniline Ti(IV)As composite material has been successfully applied for the photochemical degradation of industrial dye as well as a conducting material.
1. Introduction
Water pollution by heavy metals remains an important environmental issue associated negatively with health and economy.1 A number of chemical and biological contaminants have affected the quality of drinking water.2 Wastewaters containing toxic metals (Pb2+, Cd2+, Hg2+, Bi3+, Th4+, Zr4+etc.) are generated as a by-product from the metallurgical, galvanizing, metal finishing, electroplating, mining, power regeneration, electronic device manufacturing and tannery industries, and are found to be hazardous to health.3,4The use of lead in industry (lead-acid batteries, lead wire or pipes, metal recycling and foundries) is one of the causes of environmental contamination.5,6 Lead poisoning interferes with a variety of body processes and is toxic to many organs and tissues, causing potentially permanent learning and behaviour disorders. Children living near industrial areas that process lead, such as smelters, have been found to have unusually high blood lead levels.7 Besides human beings, plants and animals are also affected by its toxicity. The critically endangered California condor has also been affected by lead poisoning.8 Mercury accumulated in the body enters the brain cells through an oxidation reaction and turns into inorganic ions (Hg2+).9 Poisoning can result in several diseases including acrodynia, Hunter–Russell syndrome and Minamata disease.10
A number of techniques have been developed for the treatment of such waste streams. Among these, the ion-exchange method has several advantages over other methods because it is a relatively clean and energy efficient method, which also features selectivity for certain ions, even in solutions of low concentration of the target ion from industrial waste.11–13 For this purpose, inorganic ion-exchangers and organic resins have been used, but they suffer certain limitations. The main drawbacks existing with inorganic ion-exchangers are low mechanical and chemical strength and the fact that they are obtained in powdered form, which is not suitable for column applications. The serious limitations of organic resins are their poor thermal and chemical stability (less stable in highly acidic and basic media).14 Hence, in order to overcome all these barriers, there is a continuous need to investigate novel composite ion-exchangers that are capable of removing heavy metals from industrial waste.15 These show better exchange capacity, granulometric properties, reproducibility, chemical and thermal stability, and also possess good selectivity for heavy metals as compared to pure inorganic and organic materials. Polyaniline (PANI) based composite materials have been at the forefront of the global search for commercially available conducting polymers because of their unique proton dopability, low cost, ease of synthesis, excellent redox recyclability, variable electrical conductivity, and their thermal and chemical stability.16,17
Owing to the above mentioned advantages, they can be used as an ion-exchanger,18–20 a catalyst,21 an ion selective electrode,22 a conducting material23 and also find a large number of applications in pollution control and water treatment, which are of economical importance.
A column method was employed to study the sorption behaviour for the metal ions. Despite the selectivity and sensitivity of analytical techniques such as atomic absorption spectrometry, there is a crucial need for the separation and determination of trace metals from the matrix prior to its determination, due to its frequently low concentration in environmental samples. The present paper reports the synthesis and characterization of polyaniline Ti(IV)As with better thermal and chemical stability, and its application in the separation of heavy metals, photochemical degradation of dyes as well as for use as a conducting material. This paper reports significant ion exchange capacity (1.37 meq g−1) and thermal stability (78% retention of IEC up to 400 °C) compared to previous work.24
2. Experimental
2.1. Materials and methods
The main reagents for the synthesis were aniline, potassium persulfate, titanium tetrachloride, carbon tetrachloride, Tween 80 and cetyl pyridinium chloride (CPC), which were from E-Merck (India), sodium arsenate, which was from CDH (India) and Acid Blue 29 (AB 29), which was from Sigma Aldrich. All other reagents and chemicals were of analytical grade. A solution of sodium arsenate (0.25 M) was prepared in demineralized water (DMW) and a 1% solution of TiCl4 was prepared in CCl4 while a 10% solution (v/v) of aniline and 0.1 M potassium persulphate were prepared in a 1 M HCl solution. For the spectrophotometric determination of Hg(II), solutions of mercury(II) chloride (1 mg mL−1, 0.3385 g mercury(II) chloride in water and diluted to 250 mL), phenanthroline monohydrate (0.05%), acetate buffer (pH 4.5), eosin (0.05%.), EDTA (0.05 M) and gelatine (0.05%) were prepared in demineralised water and stored in an amber-coloured bottle.2.2. Apparatus
A digital pH meter Elico EL-10 (Elico, India) was used for pH measurements. A LABINDIA UV-3200 double beam spectrophotometer with 10 mm matched quartz cells was used for spectrophotometric determination. The infrared (IR) spectra were recorded on a Fourier transform-IR (FTIR) Spectrometer from Perkin Elmer (1730, USA) using the KBr disc method. Thermogravimetric analysis/differential thermal analysis (TGA/DTA) was carried out on a DTG–60 H; C305743 00134, (Schimadzu, Japan) analyzer at a rate of 10 °C min−1 in a nitrogen atmosphere. An X'Pert PRO analytical diffractometer (PW-3040/60 Netherlands with CuKα radiation λ = 1.5418 Å) was used for X-ray diffraction (XRD) measurement. A scanning electron microscopy instrument (SEM; LEO, 435 VF) was used for SEM images of the material at different magnifications. Transmission electron microscopy (TEM) analysis was carried out on a Jeol H-7500 Microscope. CHNO analysis was carried out on a Carlo Erba EA1108 (Milan, Italy) elemental analyzer. Flame atomic absorption spectrometry (FAAS) measurements were made with a Model GBC-932-Plus flame atomic absorption spectrometer (GBC Scientific, Australia). A temperature controlled shaker (MSW-275, India) was used for shaking. A muffle furnace (Narang Scientific works, India) was used for heating samples at different temperatures.2.3. Procedure for the photochemical degradation
The degradation of the composite material was carried out using a UV-vis spectrophotometer. The experiment was carried out in an immersion well photochemical reactor. For the photochemical irradiation, a 0.06 M solution of dye (AB29) was prepared in 250 mL DMW and poured into the reactor. A fixed amount of composite material (0.25 g) was added. The solution was stirred for 20 min in the dark to allow equilibration and the reading at zero time was measured from the blank solution that was otherwise treated similarly to the irradiated solution. The atmospheric oxygen was continuously supplied to the suspensions throughout the experiment. Irradiations were carried out using a 125 W medium pressure mercury lamp (radiant flux ≈ 2150 μW cm−2). Short wavelength UV and IR radiation was eliminated by circulating water in a pyrex glass jacket. The samples were collected in 10.0 mL fractions before and at regular intervals during irradiation for analysis after centrifugation. The calibration curve was obtained from the absorbance at different concentrations (mole L−1 min−1).2.4. Synthesis of polyaniline and polyaniline–Ti(IV)arsenate composite ion exchange material
Polyaniline, the inorganic precipitate (Ti(IV)As) and the polyaniline–Ti(IV)arsenate hybrid ion exchange material were synthesised using the method described in our reported paper.24 On the basis of having the highest uptake capacity and the physical appearance of the beads, together with its percentage yield, sample A-6 was selected for exhaustive studies (Table 1). The proposed structure of the composite material is shown in Scheme 1. | ||
| Scheme 1 Proposed structure of the polyaniline–Ti(IV)As cation exchanger. | ||
2.5. Synthesis of polyaniline–Ti(IV)As composite samples for the electrical measurements
For the measurement of electrical conductivity, a number of samples were prepared by adding varying amounts of inorganic precipitate (in different weight ratios of 5, 10, 15, 20, 25 wt. % of Ti(IV)As) to the polyaniline gels, using the same procedure as described above. The prepared samples of the polyaniline–Ti(IV)As composite were labeled as As-1(PANI), As-2, As-3, As-4, As-5 and As-6 (Table 2).| S.No | Aa (mol L−1) | Bb (mol L−1) | Cc (%) v/v | Mixing ratio v/v/v | Temperature | pH | Appearance of bead | IECd for Na+ ions | Yield (g) |
|---|---|---|---|---|---|---|---|---|---|
| a Sodium arsenate. b Titanium tetrachloride. c Polyaniline. d Ion exchange capacity (meq g−1). | |||||||||
| A-1 | 0.25 | 0.1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 0.5 | White granular | 0.75 | 1.62 |
| A-2 | 0.20 | 0.1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 1.0 | White granular | 0.99 | 1.96 |
| A-3 | 0.25 | 0.1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 °C | 1.0 | White granular | 0.70 | 2.01 |
| A-4 | 0.25 | 0.1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 1.5 | Green granular | 1.00 | 2.90 |
| A-5 | 0.25 | 0.1 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 0.5 | Green granular | 1.10 | 2.30 |
| A-6 | 0.25 | 0.1 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 1.0 | Green granular | 1.37 | 3.99 |
| A-7 | 0.25 | 0.1 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 °C | 1.0 | Green granular | 1.00 | 3.30 |
| A-8 | 0.25 | 0.1 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 ± 2 °C | 1.5 | White granular | 0.90 | 3.45 |
2.6. Ion exchange capacity
To determine the ion-exchange capacity, one gram of the exchanger in the H+ form was taken into a glass column (0.5 cm, internal diameter) plugged with glass wool at the bottom. The exchanger was stripped of H+ ions by allowing alkaline earth metal nitrate solutions (0.1 M) to pass through the column at a flow rate of 2.0 mL min−1. The H+ ion content of the effluent was then determined by titrating against a standard solution of sodium hydroxide (0.1 mol L−1).2.7. pH titration
In order to determine the nature of the ionogenic group of polyaniline–Ti(IV)arsenate, pH titration studies were done using the Topp and Pepper method.25 In this method, 0.5 g of the exchanger (H+ form) was taken into each of several 50 mL conical flasks, which was followed by the addition of an equimolar solution of alkali and alkaline metal chlorides and their corresponding hydroxides as NaCl–NaOH, KCl–KOH and CaCl2–Ca(OH)2, BaCl2–Ba(OH)2 systems. The final volume was adjusted to 50 mL to maintain the ionic strength.2.8. Chemical composition
The composition of the sample was determined by dissolving a 0.25 g portion of exchanger in 15 mL hot concentrated hydrochloric acid. The solution was evaporated to near dryness, then cooled and diluted to 100 mL with distilled water. Titanium and arsenate were determined spectrophotometrically using standard procedures.26,272.9. Leaching effect of arsenic
To observe the leaching effect of arsenic into the solution, a fixed amount of the material (0.3 g) was immersed into the aqueous solution of HCl, NaOH (0.1 M) and DMW (30 mL each) with occasional shaking intermittently for 24 h. The solution was filtered through a Millipore cellulose membrane filter (0.45μm pore size). The number of arsenic ions in the solution was determined by EDTA back titration using Eriochrome Black T indicator.282.10. Effect of eluent concentration
A fixed volume (250 mL) of varying concentrations of sodium nitrate solutions was used for complete elution of H+ ions from the column containing 0.5 g of the exchanger (H+ form). The effluent was titrated against the standard solution of sodium hydroxide.2.11. Elution behavior
A column containing 0.5 g of exchanger (H+ form) was eluted with 1.0 M NaNO3 solution. The effluent was collected in 10.0 mL fractions at a flow rate of 2.0 mL min−1. Each fraction of 10.0 mL was titrated against a standard solution of sodium hydroxide.2.12. Distribution (sorption) studies
The distribution coefficient (Kd) of the metal ions was determined by a batch method in non-ionic, anionic and cationic surfactant systems. The distribution coefficient is actually used to access the overall ability of the material to remove the ions of interest under set conditions. Various portions (300 mg each) of the polyaniline–Ti(IV)arsenate in the H+ form were taken into Erlenmeyer flasks and mixed with 30 mL of different metal nitrate solutions in the required medium, and subsequently shaken for 6 h in a temperature controlled shaker at 25 ± 2 °C to attain equilibrium. The metal ion concentration before and after equilibrium was determined by EDTA titration. The distribution coefficients were calculated using the equation: | (1) |

| (2) |
The sorption of metal ions involves the ion-exchange of the H+ ions in the exchanger phase with that of the metal ions in the solution phase
For example:
 | (3) |
2.13. Quantitative separations of metal ions in synthetic binary mixtures
Quantitative separations of some important metal ions were achieved on polyaniline–Ti(IV)As columns. 1 g of exchanger (H+ form) was packed in a glass column (0.5 cm, internal diameter) with a glass wool support at the end of the column. The column was washed thoroughly with demineralized water and the mixtures of two metal ions (each with initial concentration 0.1 M) were loaded onto it. It was then made to pass through the column at a flow rate of 2.0 mL min−1 until the level was just above the surface of the material. The process was repeated two or three times to ensure the complete absorption of metal ions on the bead. The separation of metal ions was achieved by collecting the effluent in 10 mL fractions and titrating against the standard solution of the di-sodium salt of EDTA (0.01 M).2.14. Selective separation of Bi3+, Hg2+ and Zr4+ from synthetic mixtures of metal ions
Selective separation of Bi3+, Hg2+ and Zr4+ from the synthetic mixtures containing (Zn2+, Ca2+, Cd2+, Mn2+, Al3+ , Bi3+ , La3+), (Mg2+, Zn2+, Ca2+, Mn2+, Hg2+, Ni2+ , Co2+) and (Mn2+, Zn2+, Sr2+, Ca2+, Co2+ Zr4+, Al3+) was achieved on polyaniline–Ti(IV)arsenate columns. The amounts of Bi2+, Hg2+ and Zr4+ ions in the synthetic mixtures was varied, keeping the amounts of the other metal ions constant.2.15. Determination of Pb2+ and Zn2+ in brass industry waste using polyaniline–Ti(IV)As columns by FAAS
2.16. Determination of Hg2+ in brass industry waste using polyaniline–Ti(IV)arsenate columns by a spectrophotometric method
3. Results and discussion
Results obtained from the synthesis of composite materials (Table 1) indicate that the ion-exchange capacity of a composite material depends upon the pH. We know from Table 1 that the ion-exchange capacity of synthesised materials decreases with increasing pH, which may be attributed to the precipitation of metal oxide at higher pH.| Samples | Stock solution (10% polyaniline) (mL) | Ti(IV)As (wt. %) | Yield (g) | Pellet thickness (mm) | Conductivity (S cm−1) |
|---|---|---|---|---|---|
| As-1 (PANI) | 100 | 0.0 | 0.290 | 0.92 | 0.028 |
| As-2 | 100 | 5 | 0.449 | 1.55 | 1.77 |
| As-3 | 100 | 10 | 0.519 | 1.20 | 3.20 |
| As-4 | 100 | 15 | 1.956 | 1.45 | 5.61 |
| As-5 | 100 | 20 | 1.640 | 1.39 | 4.31 |
| As-6 | 100 | 25 | 1.587 | 1.44 | 0.043 |
In order to investigate the working capacity of the exchanger, the ion-exchange capacities of some monovalent and divalent cations were determined (Table 3). The affinity sequence for monovalent ions was found to be K+ > Na+ > Li+ and for bivalent ions it was Mg2+ > Ca2+ > Sr2+. The ions with smaller hydrated radii easily enter the pores of the exchanger, resulting in higher adsorption.30,31 From these observations it was found that the material shows a greater exchange capacity for alkali metal ions than for alkaline earth metal ions.
For the complete removal of H+ ions from the exchanger, the optimum concentration of the eluent was found to be 1.0 M (Fig. 1). The experiment also established the minimum volume required for the complete elution of the H+ ions, which reflects the column efficiency. It was observed from Fig. 2 that the rate of exchange is quite fast in the beginning as only 60 mL NaNO3 solution (1.0 M) is sufficient for complete elution of the H+ ions from the column containing 1.0 g exchanger. From this observation it has been found that the efficiency of the column is quite satisfactory.
 | ||
| Fig. 1 Effect of eluent concentration on the ion-exchange capacity of the polyaniline–Ti(IV)As cation exchanger. | ||
The pH titration curves for each of the LiCl–LiOH, NaCl–NaOH and KCl–KOH systems show two inflexion points which infer bifunctional strong cation exchange behaviour (Fig. 3); the strong ion-exchange characteristic of this material is evident from the low pH values (<3) when initially no OH− ions are added to it. On addition of base to the metal chloride solution (in each case e.g. LiCl, NaCl and KCl) the pH increases rapidly; above pH 9 the exchanger begins to hydrolyse. It is also evident from Fig. 3 that in an acidic medium the uptake of the K+ ion is greater than that of the Na+ ion, which is greater than that of the Li+ ion.
 | ||
| Fig. 3 pH titration curves of the polyaniline–Ti(IV)As cation-exchanger with various alkali metal hydroxides. | ||
Chemical composition analysis reveals that the molar ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As
As![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H:
H:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is 1
O is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.96
3.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85
0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.68
0.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.69. The material is stable in some organic solvents (DMSO, DMF, CH3CN, m-cresol, THF) acids (HCl, HNO3, H2SO4, HClO4) and bases (NaOH, KOH) up to 2 M, while it was found to be miscible in concentrated CCl4. Thus, the exchanger is chemically resistant to most solvents and can be successfully used with diverse solvents in column operations. EDTA back titration results indicate that no arsenic ions were released from the material into the aqueous solutions of HCl, NaOH and DMW. The non leaching property of arsenic was also confirmed from the proposed structure as shown in Scheme 1, which reveals that the arsenate groups are attached to titanium by a coordinate bond, so there is no possibility of releasing the arsenic ions into the solution.
0.69. The material is stable in some organic solvents (DMSO, DMF, CH3CN, m-cresol, THF) acids (HCl, HNO3, H2SO4, HClO4) and bases (NaOH, KOH) up to 2 M, while it was found to be miscible in concentrated CCl4. Thus, the exchanger is chemically resistant to most solvents and can be successfully used with diverse solvents in column operations. EDTA back titration results indicate that no arsenic ions were released from the material into the aqueous solutions of HCl, NaOH and DMW. The non leaching property of arsenic was also confirmed from the proposed structure as shown in Scheme 1, which reveals that the arsenate groups are attached to titanium by a coordinate bond, so there is no possibility of releasing the arsenic ions into the solution.
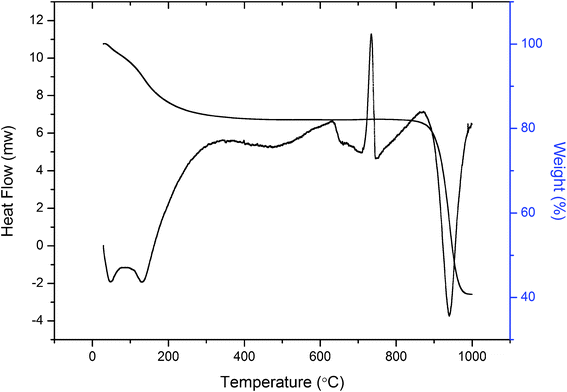
In order to get an idea of thermal stability, the material was heated for 1 h at different temperatures to determine the ion exchange capacity retained. Table 4 indicates that the ion-exchange capacity and physical appearance of the exchanger changed as the temperature increased. It was also observed that this cation-exchanger possessed improved thermal stability. However, in terms of exchange capacity, the material was found to be stable up to 300 °C, with 98% of the initial ion-exchange capacity being retained. The TGA curve of the composite material (Fig. 4) shows a continuous weight loss of about 4% up to 100 °C, which is due to the removal of external water molecules.32 The weight loss from 100 °C to 200 °C is due to the loss of interstitial water molecules by the condensation of –OH groups. Further weight loss above 816 °C is due to complete decomposition of the organic part and formation of metal oxides of the exchanger.
| Temperature/°C | Colour | % Weight loss | % Retention of IEC |
|---|---|---|---|
| 50 | Green | 0.0 | 100 |
| 100 | Green | 0.0 | 100 |
| 200 | Dark black | 0.0 | 100 |
| 300 | Dark black | 2 | 98 |
| 400 | Black and white | 36 | 78 |
| 500 | Dirty white | 44 | 55 |
| 600 | Dark grey | 75 | 47 |
| 700 | White | 76 | 18 |
Comparative FTIR spectra of polyaniline (PANI), Ti(IV)As and the composite material (polyaniline–Ti(IV)As) are shown in Fig. 5. In the spectra of polyaniline–Ti(IV)As, a strong broad band in the range 3550 to 3000 cm−1 corresponds to the presence of interstitial water and hydroxyl groups.33 A broad band at 1616 cm−1 was attributed to aquo H–O–H bending. The band at 1442 cm−1 corresponds to the presence of the –NH– of polyaniline in the composite material, while the bands with maxima at 1385, 1136 and 614 cm−1 were attributed to the in-plane bending vibration of the –CH bands.34
 | ||
| Fig. 5 FTIR spectra of the polyaniline–Ti(IV)As cation exchanger. | ||
The medium intensity broad band observed at 871 cm−1 was assigned to the υ (As–O–Ti) vibration.35 FTIR spectra of the composite material (Fig. 6) at different temperatures indicate that, on increasing the temperature, the exchange capacity sharply decreased (beyond 300 °C, Table 4) owing to the decomposition of ionogenic groups present in the material.
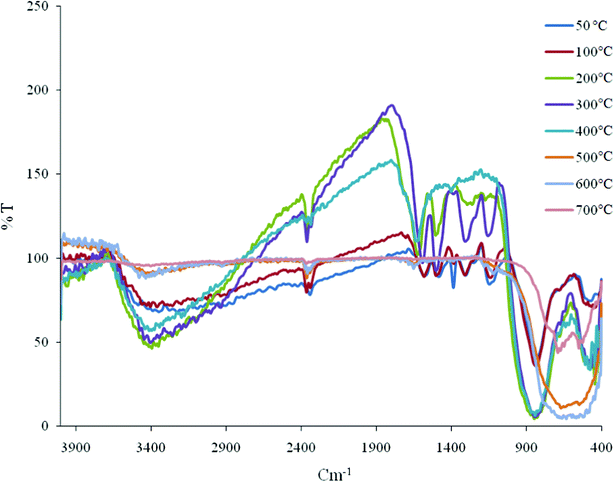 | ||
| Fig. 6 FTIR spectra of the polyaniline–Ti(IV)As cation exchanger at different temperatures. | ||
The X-ray diffraction pattern of the composite material (Fig. 7) shows some weak intensity peaks which indicate that it is slightly crystalline in nature. The SEM images obtained for polyaniline (PANI), the inorganic precipitate (Ti(IV)As) and the composite (polyaniline–Ti(IV)As, C-1) are shown at different magnifications (Fig. 8).
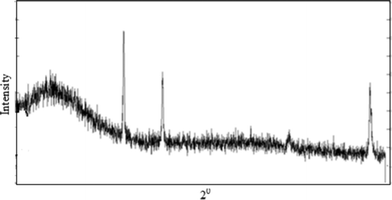 | ||
| Fig. 7 Powder X-ray diffraction pattern of the polyaniline–Ti(IV)As cation exchanger. | ||
 | ||
| Fig. 8 Scanning electron microphographs (SEM) of polyaniline (PANI), Ti(IV) arsenate (Ti(IV)As) and polyaniline–Ti(IV)As (C-1) cation exchanger. | ||
The results reveal that polyaniline and the inorganic precipitate (Ti(IV)As) have irregular zigzag particle sizes (Fig. 8, PANI, Ti(IV)As). After binding the inorganic precipitate with polyaniline, the morphology of the polyaniline–Ti(IV)As composite material has changed, and is semi-crystalline in nature (Fig. 8, C-1). TEM studies reveal that the inorganic precipitate (Ti(IV)As) and the polyaniline–Ti(IV)As composite cation exchange material (Fig. 9a, b) show particle sizes in the range of 20 nm. Thus, the material particle size shows the nano-range.
 | ||
| Fig. 9 TEM image of inorganic precipitate Ti(IV)As. Fig. 9b TEM image of inorganic precipitate polyaniline–Ti(IV)As nano-composite cation exchanger. | ||
In order to explore the potentiality of the composite cation exchange material in the separation of metal ions, distribution studies for 17 metal ions were performed in cationic (CPC), non-ionic (Tween 80) surfactants (Table 5) and mixture (solvent + surfactant) systems (Table 6). It was observed from the Kd values that the maximum sorption of Hg(II) and Bi(III) ions was found to be in 0.50% of the CPC, while the maximum sorption for Pb(II) and Zr(IV) was found to be in the mixture (Table 6). It may be due to the aggregation of counter ions around the surfactant. There are two models, which explains the distribution of counter ions around ionic micelles and association colloids. Classical electrostatic theory treats the interface as a charged surface neutralized by counter ions in the diffused electrical double layer extending radially from the aggregate surface.36
| Metal ions | Tween 80 0.25% (a) | Tween 80 0.5% (b) | Tween 80 1% (c) | Tween 80 2% (d) | CPCa 0.25% (e) | CPCa 0.50% (f) | CPCa 1% (g) | CPCa 2% (h) |
|---|---|---|---|---|---|---|---|---|
| a Cetyl pyridinium chloride | ||||||||
| Mg2+ | 11.3 | 17.6 | 2.4 | 12.2 | 33.3 | 47.1 | 0.0 | 0.0 |
| Zn2+ | 13.3 | 5.0 | 4.1 | 13.0 | 56.6 | 5.5 | 0.0 | 0.0 |
| Sr2+ | 23.0 | 34.7 | 7.3 | 11.1 | 0.0 | 32.4 | 0.0 | 0.0 |
| Ca2+ | 11.1 | 27.7 | 3.0 | 8.8 | 0.0 | 13.6 | 0.0 | 0.0 |
| Ba2+ | 54.2 | 50.0 | 70.0 | 42.8 | 0.0 | 50.0 | 0.0 | 0.0 |
| Pb2+ | 308.0 | 111.1 | 169.0 | 134.1 | 350.0 | 304.0 | 0.0 | 0.0 |
| Cd2+ | 23.5 | 37.1 | 2.1 | 3.3 | 150.0 | 7.5 | 0.0 | 0.0 |
| Mn2+ | 13.3 | 21.9 | 11.1 | 25.6 | 5.5 | 28.3 | 0.0 | 0.0 |
| Cu2+ | 46.6 | 69.2 | 58.7 | 25.0 | 105.3 | 63.0 | 0.0 | 0.0 |
| Ni2+ | 17.5 | 50.0 | 17.7 | 33.7 | 28.0 | 34.6 | 0.0 | 0.0 |
| Hg2+ | 1900 | 660.0 | 1071.0 | 900.0 | 1600.0 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420.0 420.0 |
0.0 | 0.0 |
| Co2+ | 17.6 | 37.8 | 4.1 | 6.1 | 34.6 | 31.4 | 0.0 | 0.0 |
| Al3+ | 58.3 | 53.0 | 25.7 | 78.5 | 25.0 | 63.6 | 0.0 | 0.0 |
| Fe3+ | 100.0 | 165.0 | 145.0 | 147.0 | 137.5 | 230.0 | 0.0 | 0.0 |
| La3+ | 171.4 | 102.0 | 13.8 | 200.0 | 53.2 | 40.5 | 0.0 | 0.0 |
| Bi3+ | 87.7 | 800.0 | 1700.0 | 300.0 | 80.0 | 1900.0 | 0.0 | 0.0 |
| Zr4+ | 350.0 | 450.0 | 86.6 | 233.3 | 236.0 | 365.0 | 0.0 | 0.0 |
| Metal ions | 1 M (i)a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% (j)b 1% (j)b |
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% (k)c 1% (k)c |
0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% (l)d 1% (l)d |
0.001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% (m)e 1% (m)e |
|---|---|---|---|---|---|
| a 1 M DMSO, b 1 M DMSO: 1% Tween 80, c 0.1 M DMSO: 1% Tween 80, d 0.01 M DMSO: 1% Tween 80, e 0.001 M DMSO: 1% Tween 80. | |||||
| Mg2+ | 8.8 | 1.1 | 13.3 | 3.6 | 13.3 |
| Zn2+ | 14.2 | 5.5 | 12.9 | 4.1 | 15.8 |
| Sr2+ | 16.3 | 22.2 | 264.2 | 1.98 | 15.8 |
| Ca2+ | 22.2 | 6.5 | 6.3 | 1.98 | 15.8 |
| Ba2+ | 53.1 | 42.8 | 51.7 | 37.1 | 61.5 |
| Pb2+ | 250.0 | 538.0 | 11.1 | 194.4 | 323.0 |
| Cd2+ | 22.0 | 18.3 | 32.0 | 25.8 | 24.1 |
| Mn2+ | 27.0 | 27.7 | 6.0 | 4.8 | 8.0 |
| Cu2+ | 61.4 | 62.2 | 83.3 | 35.8 | 40.0 |
| Ni2+ | 22.2 | 4.2 | 21.9 | 14.4 | 3.7 |
| Hg2+ | 1075.0 | 600.0 | 1700.0 | 3400.0 | 26.5 |
| Co2+ | 25.0 | 25.0 | 8.8 | 18.2 | 133.3 |
| Al3+ | 90.0 | 53.8 | 51.7 | 58.4 | 83.3 |
| Fe3+ | 139.0 | 66.6 | 176.3 | 152.2 | 69.5 |
| La3+ | 230.0 | 112.7 | 216.6 | 188.5 | 150.0 |
| Bi3+ | 366.6 | 25.0 | 160.0 | 400.0 | 1600.0 |
| Zr4+ | 17.6 | 1900.0 | 133.3 | 3300.0 | 200.0 |
The distribution studies show that the material was found to possess exceptionally high Kd values for Pb(II), Hg(II), Bi(III) and Zr(IV), and was hence considered to be highly selective for Pb(II), Hg(II), Bi(III) and Zr(IV). The separation capability of the material has been demonstrated by achieving a number of binary separations of some important metal ions viz. Zn(II)–Pb(II), Ca(II)–Pb(II), Cd(II)–Hg(II), Al(III)–Hg(II), Fe(III)–Zr(IV), Sr(II)–Zr(IV), Ca(II)–Zr(IV) (Table 7). The sequential elution of ions through the column depends upon the metal-eluting ligand (eluent) stability. The weakly retained metal ions get eluted first, followed by the stronger ones. The order of elution and eluents used for binary separations is also shown in Fig. 10. The separations are quite sharp and recovery was quantitative and reproducible. The Pb(II) and Zn(II) ions were determined using a flame atomic absorption spectrophotometer, while the Hg(II) metal was determined spectrophotometrically. The amounts of Pb(II) and Hg(II) (in the brass industry waste sample) were found to be 9.1 and 9.9 μg L−1, respectively, while the amounts of Zn(II) (in brass industry waste and tap water) were found to be 11.4 and 10.1 μg L−1, respectively. The practical utility of the polyaniline–Ti(IV)As composite material was demonstrated by separating Hg(II), Bi(III) and Zr(IV) from the synthetic mixtures (Tables 8–10) and Pb(II) Hg(II) and Zn(II) from industrial wastewater and tap water (Table 11).
 | ||
| Fig. 10 Chromatograms of the binary separations of the polyaniline–Ti(IV)As cation exchanger solvents used: (c) Tween 80 1%; (e) CPC 0.25%; (i) 1 M DMSO; (j) 1 M DMSO: 1% Tween 80; (k) 0.1 M DMSO: 1% Tween 80; (l) 0.01 M DMSO: 1% Tween 80; (m) 0.001 M DMSO: 1% Tween 80. Letters (c) to (m) correspond to the letters for metal ions specified in Tables 5 and 6. | ||
| S. No. | Metal ions separation | Amount loaded (mg) | Amount found (mg) | Recovery (%) | Volume of eluent used (mL) | Eluent used |
|---|---|---|---|---|---|---|
| 1 | Zn2+ | 6.53 | 6.30 | 96.50 | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% 0.1% |
| Pb2+ | 2.07 | 1.97 | 95.50 | 80 | 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
|
| 2 | Ca2+ | 4.00 | 3.88 | 97.00 | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% 0.1% |
| Pb2+ | 2.07 | 1.95 | 94.50 | 90 | 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
|
| 3 | Cd2+ | 11.24 | 10.84 | 96.50 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% 0.1% |
| Pb2+ | 2.07 | 1.97 | 95.50 | 70 | 0.1 M DMSO | |
| 4 | Cd2+ | 11.24 | 10.82 | 97.00 | 60 | 0.25% Tween 80 |
| Hg2+ | 20.05 | 19.14 | 95.50 | 70 | 0.001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
|
| 5 | Al3+ | 2.69 | 2.54 | 94.50 | 70 | 0.25% Tween 80 |
| Hg2+ | 20.05 | 18.74 | 93.50 | 70 | 0.001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
|
| 6 | Fe3+ | 5.58 | 5.35 | 96.00 | 70 | 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
| Zr4+ | 9.12 | 8.89 | 97.50 | 70 | 1% Tween 80 | |
| 7 | Sr2+ | 8.76 | 8.58 | 98.00 | 60 | 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
| Zr4+ | 9.12 | 8.84 | 97.00 | 80 | 1% Tween 80 | |
| 8 | Ca2+ | 4.00 | 3.86 | 96.50 | 60 | 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% 1% |
| Zr4+ | 9.12 | 8.84 | 97.0 | 80 | 1% Tween 80 |
| Samples | Method | Amount of Pb2+ founda (μg L−1) (% RSD)b | Amount of Hg2+ founda (μg L−1) (% RSD)b | Amount of Zn2+ founda (μg L−1) (%RSD)b |
|---|---|---|---|---|
| a Average of three replicate determinations. b % RSD, relative standard deviation; ‘–’ indicates not detected. c Recommended procedure applied without spiking. d Recommended procedure after spiking (standard addition method). | ||||
| Brass industry wastewater, Aligarh | Directc | 9.1 (3.2) | 9.9 (2.8) | 11.4 (2.8) |
| SAd | 9.3 (2.9) | 10.5 (2.2) | 11.5 (2.2) | |
| Tap water (A.M.U. Campus, Aligarh) | Direct | — | — | 10.1 (1.81) |
| SA | 4.3 (3.8) | 10.0 (2.3) | 10.0 (2.3) | |
3.1. Limit of detection and quantification
The validity of the results was tested by applying the standard addition method by spiking the water samples with a known amount (10 μg) of individual metal ions. The results pertaining to analysis of a trace amount of the metal ion of interest confirms the satisfactory recovery of the analytes. The close agreement of the results found by the direct method (applying the recommended procedure without spiking) with that found by the S. A. (standard addition after spiking) method (Table 11) indicates the reliability of the present method for metal analysis in water samples of various matrices without significant interference. The detection limit evaluated as three times the standard deviation (s) of the blank signal along with the mean blank signals (absorbance) for 15 replicate measurements were found to be 0.32 (0.0007), 0.92 (0.0019), μg L−1 for Pb(II) and Zn(II), respectively.3.2. Electrical conductivity
The data obtained for the conductivity (Table 2) of different samples of polyaniline–Ti(IV)As composites measured at 25 ± 2 °C reveal a peculiar trend. It was observed from Table 2 that as the amount of Ti(IV)As increases (up to 15%) the conductivity of the composite material increases and reaches a maximum; after that (above 15%) it decreases continuously, which may be attributed to the increasing percentage of inorganic precipitate. As evident from Scheme 1, the inorganic precipitate (Ti(IV)As) contains the –AsO4−H+ group, which is responsible for the abrupt change in conductivity. With the increase in percentage of the inorganic part, the number of –AsO4−H+ groups involved in the doping process increases, which decreases the conductivity of the composite. A slight decrease in conductivity after 15% loading can be explained due to the presence of some untreated titanium tetrachloride, which might exist in the inorganic precipitate. The range of conductivity of TiCl4 is much lower than that of Ti(IV)As, thus it might hinder the transport of the charge carrier between the polyaniline chains.Thus, there are two processes in operation which compete with each other. Firstly, there is the suppression of the electrical conductivity by the untreated TiCl4 and secondly there is the doping process by Ti(IV)As, which increases the electrical conductivity. Thus, the composite of PANI with 15% inorganic precipitate seems to have the best synergism for high conductivity. However, pure PANI possesses minimum conductivity as it has only HCl as the doping agent.37,38 A similar trend of electrical conductivity (initial increase then decrease) with increasing content of the inorganic particle (Ti(IV)As) in the matrix of polyaniline has been reported by other workers.39
3.3. Photocatalytic degradation
Photocatalytic degradation of AB-29 was investigated using the polyaniline–Ti(IV)As composite as a photocatalyst at 25 ± 2 °C. Fig. 11 shows that in the absence of the composite no change was observed in the concentration of the dye; however, in the presence of polyaniline–Ti(IV)As, the concentration of the dye decreases as a function of irradiation time, which implies that photocatalytic degradation of AB-29 takes place in the presence of polyaniline–Ti(IV)As, which is acting as a photocatalyst in the aqueous suspension of the polyaniline–Ti(IV)As composite.40 | ||
| Fig. 11 Change in concentration as a function of time for irradiation of an aqueous suspension of acid blue (AB 29) in the presence and absence of a photocatalyst (polyaniline–Ti(IV)As), irradiation time = 180 min. | ||
4. Conclusion
The semi-crystalline polyaniline–Ti(IV)As nano-composite cation exchanger shows selective behaviour towards heavy metal ions and can withstand fairly high temperatures. Thermally stable, it retains significant ion-exchange capacity up to 300 °C. It can be used for the quantitative separation of metal ions from a binary mixture of analytical importance. Determination of Pb(II) and Zn(II) ions from tap water and industrial wastewater samples by FAAS does not require any prior digestion. Thus, the polyaniline–Ti(IV)As nano-composite cation exchanger exhibits the characteristics of a promising ion-exchanger, which can be explored for other applications. Electrical conductivity measurements and photochemical degradation results indicate that the composite material can be used as a conducting material as well as a photocatalyst for the degradation of a textile industry dye.Acknowledgements
We gratefully acknowledge the financial support provided by the Council of Science and Technology, Lucknow, U.P. under the research scheme No. CST/D-3554.References
- J. V. Oostdam, S. G. Donaldson, M. Feeley, D. Arnold, P. Ayotte, G. Bondy, L. Chan, E. Dewaily, C. M. Furgal, H. Kuhnlein, E. Loring, G. Muckle, E. Myles, O. Receveur, B. Tracy, U. Gill and S. Kalhok, Sci. Total Environ., 2005, 351, 165 CrossRef.
- T. Pradeep and Anshup, Thin Solid Films, 2009, 517, 6441 CrossRef.
- S. Rengaraj and C. K. Joo, J. Hazard. Mater., 2003, 102, 257 CrossRef CAS.
- A. M. Khan, S. A. Ganai and A. Nabi, Colloids Surf., A, 2008, 337, 141 CrossRef.
- P. Ragan and T. Turner, JAAPA, 2009, 22, 40 Search PubMed.
- N. Manay, Az. Cousillas, C. Alvarez and T. Heller, Rev. Environ. Contam. Toxicol., 2008, 195, 93 CAS.
- M. D. Sanborn, A. Abelsohn, M. Campbell and E. Weir, CMAJ, 2002, 166, 1287 Search PubMed.
- M. E. Finkelstein, D. G. Eorge, S. Cherbinski, R. G. Wiazda, M. Johnson, J. Burnett, J. Brandt, S. Lawrey, A. P. Pessier, M. Clark, O. J. Wynne, O. J. Grantham and D. R. Smith, Environ. Sci. Technol., 2010, 44, 2639 CrossRef CAS.
- J. C. Clifton, Pediatr. Clin. North Am., 2007, 54, 237 CrossRef.
- P. W. Davidson, G. J. Myers and B. Weiss, Pediatrics., 2004, 113, 1023 Search PubMed.
- M. N. Akieh, M. Lahtinen, A. Vaisanen and M. Sillanp, J. Hazard. Mater., 2008, 152, 640 CrossRef CAS.
- S. Bag, P. N. Trikalitis, P. J. Chupas, G. S. Armatas and M. G. Kanatzidis, Science, 2007, 317, 490 CrossRef CAS.
- M. J. Manos, C. D. Malliakas and M. G. Kanatzidis, Chem.–Eur. J., 2006, 13, 51 CrossRef.
- W. A. Siddiqui and S. A. Khan, Colloids Surf., A, 2007, 295, 193 CrossRef CAS.
- S. A. Nabi, A. H. Shalla, A. M. Khan and S. A. Ganie, Colloids Surf., A, 2007, 302, 241 CrossRef CAS.
- H. S. O. Chan, S. C. Ng, W. S. Sim, S. H. Seow, K. L. Tan and B. T. G. Tan, Macromolecules, 1993, 26, 144 CrossRef CAS.
- Y. Wei, R. Hariharan and S. A. Patel, Macromolecules, 1990, 23, 758 CrossRef CAS.
- O. Arrad and Y. Sasson, J. Org. Chem., 1989, 54, 4993 CrossRef CAS.
- S. A. Nabi, Md. Shahadat, R. Bushra, A. H. Shalla and A. Azam, Colloids Surf., B, 2011, 87, 122 CrossRef CAS.
- S. A. Nabi, Md. Shahadat, R. Bushra, A. H. Shalla and F. Ahmed, Chem. Eng. J., 2010, 165, 405 CrossRef CAS.
- O. M. Vatutsina, V. S. Soldatov, V. I. Sokolova, J. Johann, M. Bissen and A. Weissenbacher, React. Funct. Polym., 2007, 67, 184 CrossRef CAS.
- S. A. Nabi, A. H. Shalla and S. A. Ganai, Sep. Sci. Technol., 2008, 43, 164 CrossRef CAS.
- M. A. Hafez, M. M. Kenway, M. A. Akl and R. R. Lshein, Talanta, 2001, 53, 749 CrossRef CAS.
- S. A. Nabi, Md. Shahadat, R. Bushra and A. H. Shalla, Chem. Eng. J., 2011, 175, 8 CrossRef CAS.
- N. E. Topp and K. W. Pepper, J. Chem. Soc., 1949, 3299 RSC.
- F. D. Snell, C. T. Snell, Calorimetric Methods of Chemical Analysis, vol. II, D. Van Nostrand, NJ, 1959, pp. 325 Search PubMed.
- F. D. Snell, C. T. Snell, Calorimetric Methods of Chemical Analysis, vol. II, D. Van Nostrand, N.J., 1959, pp. 334 Search PubMed.
- F. J. Welcher, The Analytical Uses of Ethylene Diamine Tetra Acetic Acid, Princeton New Jersey., 1957, 254 Search PubMed.
- J. R. Mudakavi, Analyst, 1984, 109, 1577 RSC.
- A. A. Khan, Inamuddin and M. M. Alam, React. Funct. Polym., 2005, 63, 119 CrossRef CAS.
- S. A. Nabi, S. Usmani and N. Rehman, React. Funct. Polym., 2006, 66, 495 CrossRef CAS.
- C. Duval, Inorganic Thermogravimetric Analysis, Elsevier, Amsterdam, 1963, p. 315 Search PubMed.
- C. N. R. Rao, Chemical Application of Infrared spectroscopy, Academic Press, New York, 1963, p. 355 Search PubMed.
- R. M. Silverstein, G. C. Bassler and T. C. Morrill, Spectrometric identification of organic compounds, John Wiley and Sons, New York, 4th edn, 1981, ch. 3, p. 111 Search PubMed.
- M. Zhang, Guangzhi He and G. Pan, J. Colloid Interface Sci., 2009, 338, 284 CrossRef CAS.
- G. Gunnarsson, B. Jonsson and H. Wennerstrom, J. Phys. Chem., 1980, 84, 3114 CrossRef CAS.
- J. C. Xu, W. M. Liu and H. L. Li, Mater. Sci. Eng., C, 2005, 25, 444 CrossRef.
- S. J. Su and N. Kuramoto, Synth. Met., 2000, 114, 147 CrossRef CAS.
- M. O. Ansari and F. Mohammad, Sens. Actuators, B, 2011, 157, 122 CrossRef CAS.
- M. Faisal, M. Abu Tariq, A. Khan, K. Umar and M. Muneer, Sci. Adv. Mater., 2011, 3, 269 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |