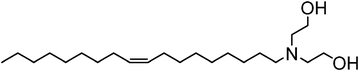
Bio-derived oleyl surfactants as porogens for the sustainable synthesis of micelle-templated mesoporous silica†
Christian P.
Canlas
and
Thomas J.
Pinnavaia
*
Department of Chemistry, Michigan State University, East Lansing, Michigan 48823. E-mail: pinnavaia@chemistry.msu.edu
First published on 13th July 2012
Abstract
There is a growing trend towards “greener” alternatives to the synthesis of materials through the use of renewable chemical resources. In this study, we report the utilization of plant-derived oleyl amine surfactants as porogens for the synthesis of mesoporous silicas in place of the usual alkyl amine surfactants. Oleyl–NH2 and oleyl–NH–(CH2)3NH2 derivatives are shown to be effective templating agents for the hydrogen bonded assembly of mesoporous silicas with well expressed wormhole and lamellar framework structures. Also, under appropriate reaction conditions these surfactants are capable of templating mesoporous silica with framework pore size within the supermicroporous range through solvent polarity modification.
I. Introduction
The micelle-directed synthesis of mesoporous silica1,2 generally is achieved through two reaction pathways, namely, the electrostatic assembly pathway and the electrically neutral pathway. The electrostatic S+I− pathway utilizes charge matching interactions between the cationic surfactant (S+), such as a quaternary ammonium cation, and an anionic inorganic silica precursor (I−), such as a silicate ion. This type of assembly mechanism is used, for example, in the synthesis of MCM-41 (1D hexagonal), MCM-48 (cubic) and MCM-50 (lamellar).2,3 A variation of this synthetic pathway, denoted the electrostatic S+X−I+ pathway, involves an assembly of reagents utilizing interactions between a cationic surfactant (S+) and a cationic silica precursor (I+) that is mediated by a counter ion (X−). SBA-15 silica (2D hexagonal) is prepared via an S+X−I pathway.4 The charge balancing forces of the electrostatic pathway generally afford long range structures with regular pore diameters, uniform pore wall thicknesses, and specific space group symmetries, even though the pore walls are atomically disordered (amorphous).The electrically neutral assembly pathway, denoted S0I0, utilizes hydrogen bonding interactions between a neutral surfactant (S0) and an uncharged silica precursor (I0).5 The comparatively shorter range hydrogen bonding forces result in structures with uniform pores and wall thickness, but with lower framework structures that have been described as lamellar to wormhole-like. Such structures typically exhibit a one or two low angle X-ray diffraction peaks corresponding to a pore-to-pore correlation length. Despite the limited framework regularity afforded by the S0I0 assembly pathway, mesoporous silicas assembled from electrically neutral amine5,6 or block copolymer surfactants7 exhibit surface areas comparable to those made by electrostatic assembly (e.g., ∼1000 m2 g−1) and pore diameters range from 3–5 nm.
Virtually all silica mesophases prepared through both electrostatic and electrically neutral reaction pathways have been prepared to date through the use of surfactants in which the saturated hydrophobic segment of the surfactant molecule is derived from petroleum (e.g., the polypropylene oxide segments of block co-polymer surfactants) or from tallow fats and vegetable oil sources (e.g., saturated C12 lauryl, C16 palmityl and C18 stearyl segments). Recently, there has been growing interest in “greener” approaches to the synthesis of materials, in general. As the number of number of potential applications for mesoporous silica and related metal oxides continues to grow,8–12 there will be an increasing need to utilized surfactant porogens in which the hydrocarbon-rich hydrophobic segment of the molecule is derived from renewable vegetable sources. Herein, we describe the use of unsaturated C18 oleyl amine surfactants for the synthesis of mesoporous silicas with wormhole framework structures. Oleyl amine is derived through a number of different possible processing routes from esters of oleic acid,13,14 which are found in relative abundance in soya and palm oils. Notably, oleyl amine was recently shown to be a promising capping agent in the synthesis of various nanoparticles.15,16 We demonstrate here that oleyl amines also are promising porogen reagents for the synthesis of mesoporous silicas through an electrically neutral hydrogen bonding pathway.
II. Experimental
Reagents
Bis(2-hydroxyethyl)oleylamine (oleyl-N(CH2CH2OH)2), oleylamine (oleyl–NH2), oleyldimethylamine (oleyl–N(CH3)2), and N-oleyl-1,3-diaminopropane (oleyl–NH(CH2)3NH2) were provided by Akzo Nobel and used without further purification. Tetraethylorthosilicate (TEOS), and dodecylamine (DDA) were purchased from Aldrich. Absolute ethanol was purchased in-house. All water used in the experiments was double exchanged using a Millipore filter apparatus to remove cations and anions.Mesostructure synthesis
Table 1 provides the structures of the oleyl surfactants used for the assembly of mesoporous silica. The procedures used for the oleyl amine-templated synthesis of mesoporous silica via the S°I° pathway are analogous to those described in the literature for the preparation of mesoporous silicas made from alkyl amine porogens.17 Since the textural properties of silica mesophases are known to be affected by differences in solvent polarity and assembly temperature, the reactions were carried out in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures over the range of 10
water mixtures over the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 to 100
90 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (v/v) and at temperatures of 25, 45 and 60 °C. In a typical synthesis TEOS (10 g, 0.048 mol) was added to a solution of the desired amine surfactant (0.013 mol) dissolved in 60 mL of a mixture of ethanol and water in 250-mL polypropylene bottles. The reaction mixture was aged, with stirring, at the desired reaction temperature for 20 h. The products were recovered by filtration and air dried. The surfactant template was removed by calcination at 600 °C for 4 h at a heating rate of 2 °C per minute.
0 (v/v) and at temperatures of 25, 45 and 60 °C. In a typical synthesis TEOS (10 g, 0.048 mol) was added to a solution of the desired amine surfactant (0.013 mol) dissolved in 60 mL of a mixture of ethanol and water in 250-mL polypropylene bottles. The reaction mixture was aged, with stirring, at the desired reaction temperature for 20 h. The products were recovered by filtration and air dried. The surfactant template was removed by calcination at 600 °C for 4 h at a heating rate of 2 °C per minute.
Physical measurements
The physical properties of the mesostructured silica reaction products were determined by nitrogen adsorption isotherms, powder X-ray diffraction and transmission electron microscopy.Nitrogen physisorption isotherms were obtained at −196 °C on a Micromeritics Tristar 3000 sorptometer. The calcined samples were out-gassed overnight at 150 °C and 10−3 torr.
Powder X-ray diffraction patterns were obtained using Cu-Kα radiation (λ = 1.542 Å) and a Rigaku Rotaflex diffractometer equipped with a rotating anode operated at 45 kV and 100 mA. Counts are accumulated every 0.02° 2θ at a scan speed of 0.5° min−1.
Transmission electron microscopy (TEM) images were obtained on a JEOL 2200FS with an accelerating voltage of 200 kV. Samples are prepared by sonicating the powdered samples in ethanol for 15 min and subsequently evaporating two drops of the suspension onto a carbon coated film supported on a 3 mm, 300 mesh copper grid.
III. Results and discussion
In general, the micelle-directed assembly of mesoporous silicas via the S0I0 pathway is dependent not only on the nature of the amine surfactant, but also on the temperature and polarity of the solvent used to carry out the assembly process.17,18 In order to assess the structure-directing viability of the oleyl surfactants shown in Table 1, we conducted initial survey experiments wherein the silica to surfactant mole ratio was held at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27 and the polarity of the water
0.27 and the polarity of the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio was either a comparatively low polarity ratio of 40
ethanol ratio was either a comparatively low polarity ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (v/v) or a higher polarity ratio of 70
60 (v/v) or a higher polarity ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v). Fig. 1 provides the low angle X-ray powder diffraction (XRD) patterns for the products assembled at 25 °C from the more polar 70
30 (v/v). Fig. 1 provides the low angle X-ray powder diffraction (XRD) patterns for the products assembled at 25 °C from the more polar 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 water
30 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solvent mixture. Included in the Fig. 1 is the powder pattern for the product templated under analogous conditions from dodecylamine, a known petroleum-derived structure director of wormhole or lamellar framework structures.5,19 Analogous X-ray patterns were obtained for the products assembled from the less polar 40
ethanol solvent mixture. Included in the Fig. 1 is the powder pattern for the product templated under analogous conditions from dodecylamine, a known petroleum-derived structure director of wormhole or lamellar framework structures.5,19 Analogous X-ray patterns were obtained for the products assembled from the less polar 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 water
60 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solvent.
ethanol solvent.
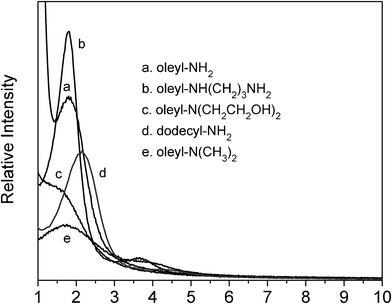 | ||
Fig. 1 Low angle powder XRD patterns for calcined silicas prepared from amine surfactants in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) water 30 (v/v) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol reaction medium at 25 °C. ethanol reaction medium at 25 °C. | ||
As indicated by the XRD patterns in Fig. 1, all four oleyl surfactants afford calcined products that exhibit a broad low angle line consistent with the presence of a wormhole framework structure. In fact, the products templated by the oleyl–NH2 and –NH(CH2)3NH2 surfactants are well expressed, as judged from the intensity of the low angle diffraction peaks. However, the N-alkylated oleylamines with compromised hydrogen bonding ability (i.e., oleyl–N(CH3)2 and oleyl–N(CH2CH2OH)2) exhibit weak and relatively broad diffraction peaks indicative of a compromised mesostructure formation.
The nitrogen adsorption data for the products obtained in our initial survey experiments are consistent with the results of the XRD data. As shown by the nitrogen adsorption isotherms in Fig. 2, the products templated by oleyl–NH2 and oleyl –NH(CH2)3NH2 surfactants, as well as the product formed from dodecyl–NH2, exhibit well-expressed mesopore filling steps at partial pressures between 0.30 and 0.60. The products made from the weaker hydrogen bonding oleyl–N(CH3)2 and oleyl–N(CH2CH2OH)2 porogens, however, do not show the presence of regular mesopores. All of the reaction products exhibit the presence of inter-particle textural mesopores, as evidenced by the abrupt uptake of nitrogen above a partial pressure of 0.80. These textural pores arise from the aggregation of small primary silica particles, not through surfactant templating. Similar nitrogen adsorption isotherms were obtained for the calcined forms of the silicas assembled in the lower polarity medium containing a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (v/v) ratio of water
60 (v/v) ratio of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol.
ethanol.
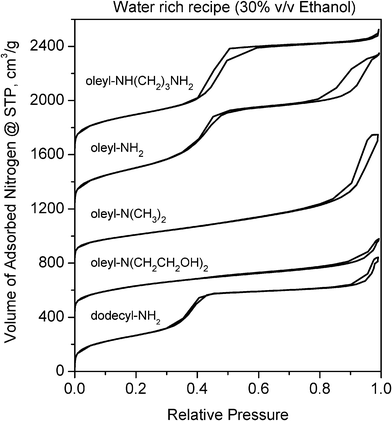 | ||
Fig. 2 Nitrogen isotherms for calcined silicas prepared at 25 °C in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) water 30 (v/v) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol in the presence of amine surfactants. The isotherms are offset by 400 cm3 g−1. ethanol in the presence of amine surfactants. The isotherms are offset by 400 cm3 g−1. | ||
Table 2 summarizes the structural information obtained from the nitrogen adsorption and XRD data. The surface areas of the mesophases that exhibit well expressed small angle diffraction are generally larger in comparison to products that exhibit weak low angle diffraction. It is noted that the ordered products made from the oleyl –NH2 and –NH(CH2)3NH2 surfactants have larger d-spacings and pore diameters in comparison to the silica template by dodecyl–NH2. This observation is consistent with the longer chain length of the oleyl amine porogens. It also is noteworthy that the lower polarity 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 water
60 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol medium is compatible with mesophase formation in the case of oleyl–N(CH3)2, whereas little mesophase formation is observed for this surfactant template in the higher polarity 70
ethanol medium is compatible with mesophase formation in the case of oleyl–N(CH3)2, whereas little mesophase formation is observed for this surfactant template in the higher polarity 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 reaction medium. This indicated that polarity matching between the surfactant head group and the reaction medium is important in determining the ability of the surfactant to template a mesophase via a hydrogen bonding mechanism. Nevertheless, there is no structural advantage to using the N-alkylated oleylamines in favor of the oleyl surfactants containing –NH2 and –NH(CH2)3NH2 head groups.
30 reaction medium. This indicated that polarity matching between the surfactant head group and the reaction medium is important in determining the ability of the surfactant to template a mesophase via a hydrogen bonding mechanism. Nevertheless, there is no structural advantage to using the N-alkylated oleylamines in favor of the oleyl surfactants containing –NH2 and –NH(CH2)3NH2 head groups.
| Surfactant | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio (v/v) ethanol ratio (v/v) |
d-Spacing (nm) | Pore sizea BJH (nm) | Wall Thicknessb (nm) | Surface Area (m2 g−1) | Framework Pore Volumec (cm3 g−1) | Total Pore Volumed (cm3 g−1) |
|---|---|---|---|---|---|---|---|
| a Obtained from the adsorption leg of the nitrogen isotherm. b Obtained from the difference between the d-spacing and average pore diameter. c The framework pore was determined at a partial pressure of 0.80. d The total pore volume was obtained at a partial pressure of 0.95. e Very weak and broad low angle diffraction intensity. | |||||||
| Oleyl–NH2 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
4.70 | 3.0 | 1.70 | 1165 | 1.28 | 1.71 |
| Oleyl–N(CH3)2 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
5.32 | – | – | 801 | – | 0.93 |
| Oleyl–N(CH2CH2OH)2 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
5.26e | – | – | 795 | – | 1.47 |
| Oleyl–NH(CH2)3NH2 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
4.46e | 3.1 | 1.36 | 1196 | 1.24 | 1.47 |
| DDA | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
4.13 | 2.6 | 1.53 | 1012 | 0.95 | 1.32 |
| Oleyl–NH2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
4.17 | 2.5 | 1.67 | 1008 | 0.89 | 0.93 |
| Oleyl–N(CH3)2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
4.46e | – | – | 1151 | 0.85 | 1.24 |
| Oleyl–N(CH2CH2OH)2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
5.45e | – | – | 740 | – | 1.32 |
| Oleyl–NH(CH2)3NH2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
4.42 | 2.8 | 1.62 | 889 | 0.82 | 1.01 |
| DDA | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
4.02 | 2.5 | 1.52 | 984 | 1.28 | 1.47 |
In view of the results of the above pilot study, we further investigated the effect of solvent polarity and assembly temperature on the textural properties of silica mesophases templated by oleyl–NH2 and oleyl–NH(CH2)3NH2 porogens. The reaction medium was varied over the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol composition range 100
ethanol composition range 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 10
0 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 and the assembly reactions were carried out at three temperatures, namely, 25, 45 and 60 °C. The polarity of the reaction medium and the reaction temperature are both expected to mediate the H-bonding interactions between the amine head group and the walls of the mesostructure, and thereby affect the textural properties of the resulting mesostructures.
90 and the assembly reactions were carried out at three temperatures, namely, 25, 45 and 60 °C. The polarity of the reaction medium and the reaction temperature are both expected to mediate the H-bonding interactions between the amine head group and the walls of the mesostructure, and thereby affect the textural properties of the resulting mesostructures.
Table 3 provides the designation for the reaction products and the corresponding reaction conditions used to prepare the mesoporous silicas templated by the oleyl–NH2 and oleyl–NH(CH2)3NH2 surfactants. The XRD powder patterns for these products are provided in Fig. 3 and 4, respectively. With the exception of the A-10-60 product made in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (v/v) water
90 (v/v) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol at 60 °C, the products templated by oleyl–NH2 over the temperature range 25°–60 °C all are mesostructured, as evidenced by the presence of at least one low angle XRD peak. Similarly, oleyl-NH(CH2)3NH2 also affords mesostructured products under analogous conditions, except for the D-100-25 product formed in 100% water at 25 °C. The oleyl–NH2 surfactant generally forms products that exhibit a single low angle XRD peak characteristic of a wormhole framework structure. Wormhole frameworks have pores and pore walls of uniform dimensions, but the pores are not spatially ordered. On the other hand, oleyl–NH(CH2)3NH2 affords primarily products that exhibit an intense first order reflection and a broader and weaker second order peak consistent with a lamellar pore structure. (cf., Fig. 4). The assignment of predominantly wormhole and lamellar pore structures templated by oleyl–NH2 and oleyl–NH(CH2)3NH2, respectively, is confirmed by the TEM images shown in Fig. 5 for products A50-60 and D50-60. The dependence of the mesopore structure on the nature of the polar head group, the assembly temperature and the polarity of the solvent medium attest to the importance of solvation effects on the hydrogen bonding interactions that influence the outcome of the S0I0 supramolecular assembly process.
ethanol at 60 °C, the products templated by oleyl–NH2 over the temperature range 25°–60 °C all are mesostructured, as evidenced by the presence of at least one low angle XRD peak. Similarly, oleyl-NH(CH2)3NH2 also affords mesostructured products under analogous conditions, except for the D-100-25 product formed in 100% water at 25 °C. The oleyl–NH2 surfactant generally forms products that exhibit a single low angle XRD peak characteristic of a wormhole framework structure. Wormhole frameworks have pores and pore walls of uniform dimensions, but the pores are not spatially ordered. On the other hand, oleyl–NH(CH2)3NH2 affords primarily products that exhibit an intense first order reflection and a broader and weaker second order peak consistent with a lamellar pore structure. (cf., Fig. 4). The assignment of predominantly wormhole and lamellar pore structures templated by oleyl–NH2 and oleyl–NH(CH2)3NH2, respectively, is confirmed by the TEM images shown in Fig. 5 for products A50-60 and D50-60. The dependence of the mesopore structure on the nature of the polar head group, the assembly temperature and the polarity of the solvent medium attest to the importance of solvation effects on the hydrogen bonding interactions that influence the outcome of the S0I0 supramolecular assembly process.
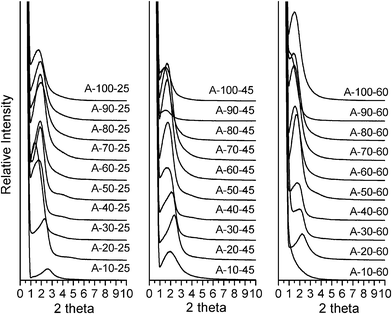 | ||
Fig. 3 Powder XRD patterns of calcined silica products prepared in the presence of oleyl–NH2 as the porogen at reaction temperatures of 25, 45, and 60 °C and in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol reaction media containing 10 to 100% water by volume. ethanol reaction media containing 10 to 100% water by volume. | ||
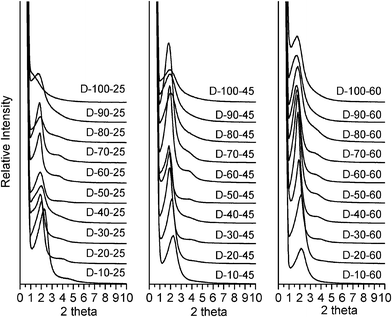 | ||
Fig. 4 Powder XRD patterns of calcined silica products prepared in the presence of oleyl–NH(CH2)3NH2 as the porogen at reaction temperatures of 25, 45, and 60 °C and in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol reaction media containing 10 to 100% water by volume. ethanol reaction media containing 10 to 100% water by volume. | ||
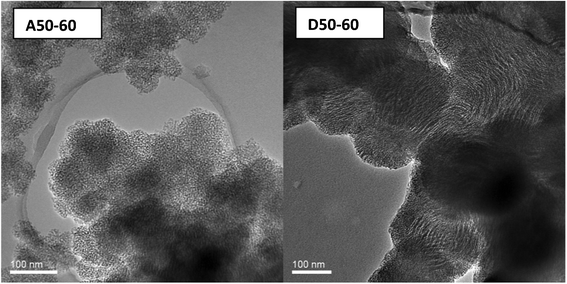 | ||
Fig. 5 TEM images of representative wormhole (A50-60) and lamellar (D50-60) framework structures for mesoporous silicas templated by oleyl–NH2 and oleyl–NH(CH2)3NH2 porogens, respectively, in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) water 50 (v/v) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol media at 60 °C ethanol media at 60 °C | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol media of different polarities
ethanol media of different polarities
| Product designationa | Synthesis T (°C) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (v/v) EtOH (v/v) |
|---|---|---|
| a In the X-Y-Z notation, X is assigned an “A” or a “D” for products templated by oleyl–NH2 or oleyl–NH(CH2)3NH2, respectively; Y is the volume % H2O in the reaction medium and Z provides the assembly temperature. | ||
| X-10-Z | 25, 45, 60 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
| X-20-Z | 25, 45, 60 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
| X-30-Z | 25, 45, 60 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
| X-40-Z | 25, 45, 60 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
| X-50-Z | 25, 45, 60 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| X-60-Z | 25, 45, 60 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| X-70-Z | 25, 45, 60 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| X-80-Z | 25, 45, 60 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| X-90-Z | 25, 45, 60 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| X-100-Z | 25, 45, 60 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Nitrogen adsorption–desorption isotherms and BJH pore distributions for representative products synthesized at 60 °C from oleyl–NH(CH2)3NH2 micelles as structure directors are presented in Fig. 6. Table 4 summarizes the textural properties of these five representative derivatives. The textural properties of each of the 60 reaction products prepared in this study (cf., Table 3) are provided as supplementary information in Table S1 and Table S2.†
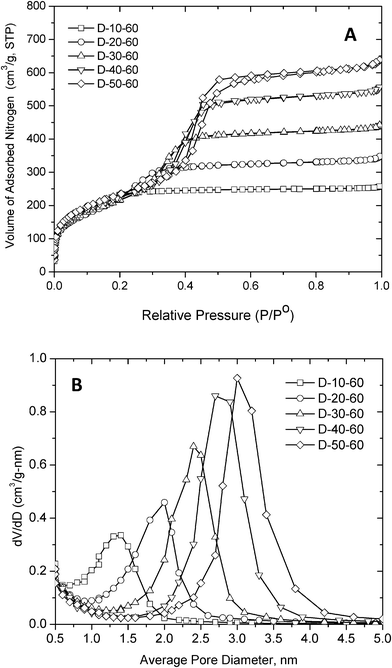 | ||
Fig. 6 (A). Nitrogen adsorption isotherm of mesostructured products templated using oleyl–NH(CH2)3NH2 as the porogen at an assembly temperature of 60oC and in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol reaction media containing 10–50 volume % water. (B) BJH pore distribution obtained from the adsorption legs of the corresponding isotherms. ethanol reaction media containing 10–50 volume % water. (B) BJH pore distribution obtained from the adsorption legs of the corresponding isotherms. | ||
| Product designation | d-Spacing (nm) | Average pore diameter (nm) | BET Surface area (m2 g−1) | Framework pore volume (mL g−1) (P/Po = 0.75) |
|---|---|---|---|---|
| D-10-60 | 4.2 | 1.4 | 738 | 0.39 |
| D-20-60 | 4.2 | 2.0 | 910 | 0.51 |
| D-30-60 | 4.6 | 2.4 | 880 | 0.66 |
| D-40-60 | 4.9 | 2.8 | 826 | 0.82 |
| D-50-60 | 5.0 | 3.2 | 834 | 0.93 |
As can be seen from the isotherms in Fig. 6 and the textural properties in Table 4, the D-10-60 product assembled in the low polarity 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (v/v) water
90 (v/v) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solvent exhibits nitrogen uptake curves indicative of the presence of supermicropores with an average diameter of 1.4 nm. Increasing the water content of the reaction medium corresponding to water
ethanol solvent exhibits nitrogen uptake curves indicative of the presence of supermicropores with an average diameter of 1.4 nm. Increasing the water content of the reaction medium corresponding to water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratios of 20
ethanol ratios of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 and beyond introduces 2.0–3.2 nm mesopores, as evidenced by the stepwise uptake of nitrogen at partial pressures between 0.30 and 0.50 (cf., Fig. 6B and Table 4). Increasing the water
80 and beyond introduces 2.0–3.2 nm mesopores, as evidenced by the stepwise uptake of nitrogen at partial pressures between 0.30 and 0.50 (cf., Fig. 6B and Table 4). Increasing the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio beyond a 50
ethanol ratio beyond a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio does not further alter the framework pore properties appreciably. However, very high water concentrations (e.g., 80–100 volume % water) promote the formation of spongelike particles, as opposed to the more monolithic particles prepared at lower water concentrations. The spongelike nature of these latter products gives rise to textural mesopores, in addition to micelle-templated framework pores.
50 ratio does not further alter the framework pore properties appreciably. However, very high water concentrations (e.g., 80–100 volume % water) promote the formation of spongelike particles, as opposed to the more monolithic particles prepared at lower water concentrations. The spongelike nature of these latter products gives rise to textural mesopores, in addition to micelle-templated framework pores.
We note that oleyl surfactants have 18 carbon atoms in the hydrophobic segment. An extended chain conformation about the cis double bond at carbon number 9 (see Fig. 7) is expected to produce micelles with a diameter of about 3–3.5 nm, which is consistent with the observed mesopore sizes for the derivatives formed in the more hydrophilic reaction media. However, the smaller supermicroporous derivatives formed in low polarity media suggest that the hydrophobic region of the surfactant may adopt a hairpin conformation (cf.Fig. 7). This flexibility in hydrophobic chain conformation may explain the dependence of framework pore size on solvent polarity. We also note that other unsaturated C18 hydrophobic moieties found in vegetable oils, (e.g., linoleic and linolenic esters with two and three cis double bonds in the C18 chain, respectively) would likely afford different pore sizes owing to differences in chain conformation and micelle size in comparison to the oleyl hydrophobe.
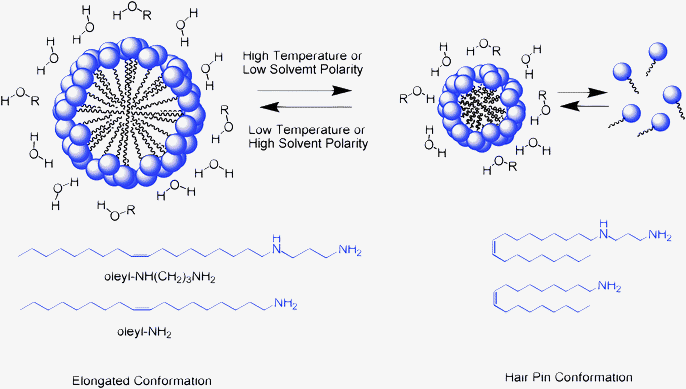 | ||
| Fig. 7 Schematic illustration of the effect of conformational changes in the hydrophobic segment of oleyl surfactants on the size of the templating micelles. | ||
The textural properties of the silica mesophases templated by oleyl surfactants are similar, though not identical, to those provided by a saturated amine surfactant containing the same number of carbon atoms. Fig. 8 provides the nitrogen adsorption isotherms and BJH pore size distributions for mesophases prepared in different water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol media at 25 °C in the presence of tallow amine (TA) as the templating agent. As in the case of petroleum derived amine surfactants, this saturated 18-carbon surfactant under comparable conditions affords mesophases with average pore sizes in the small mesopore range 2–4 nm.2,6,17 However, unlike oleyl–NH2, which is capable of templating microporous (1.2–1.8 nm) silica in lower polarity media (e.g.,< 30 vol % water), TA and related saturated amines exclusively afford products with mesopores under equivalent reaction conditions. Apparently, the saturated hydrophobic segment of TA does not adopt a smaller hairpin conformation, regardless of the polarity of the reaction medium.
ethanol media at 25 °C in the presence of tallow amine (TA) as the templating agent. As in the case of petroleum derived amine surfactants, this saturated 18-carbon surfactant under comparable conditions affords mesophases with average pore sizes in the small mesopore range 2–4 nm.2,6,17 However, unlike oleyl–NH2, which is capable of templating microporous (1.2–1.8 nm) silica in lower polarity media (e.g.,< 30 vol % water), TA and related saturated amines exclusively afford products with mesopores under equivalent reaction conditions. Apparently, the saturated hydrophobic segment of TA does not adopt a smaller hairpin conformation, regardless of the polarity of the reaction medium.
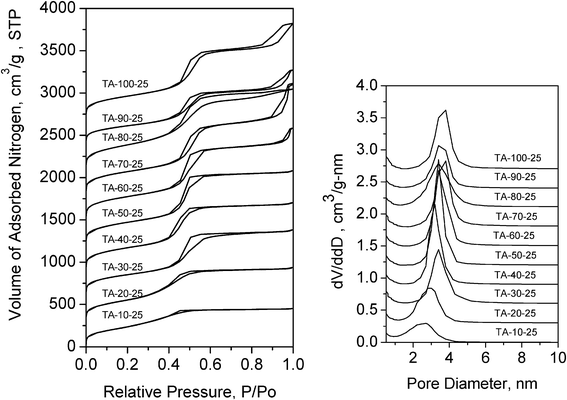 | ||
Fig. 8 Nitrogen isotherms (left panel) and BJH pore size distributions (right panel) of wormhole silicas templated by tallow amine (TA) at 25 °C in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol media containing 10 to 100% (v/v) water. ethanol media containing 10 to 100% (v/v) water. | ||
IV. Conclusions
The results obtained in this study demonstrate that renewable oleyl amine surfactants containing –NH2 and –NH(CH2)3NH2 head groups are effective agents for templating mesoporous silica with wormhole and lamellar framework structures. N-alkylation of the head groups compromises the hydrogen bond forces needed for templating via the S0I0 pathway. Assembly temperatures in excess of 60 °C also compromise hydrogen interactions at the surfactant–silica interface and lead to products lacking a well expressed mesostructure. Importantly, products templated by oleyl–NH2 and oleyl–NH(CH2)3NH2 exhibit mesopore size distributions and related textural properties comparable to those of mesophases templated by saturated amine surfactants.Overall, the assembly of wormhole silica using oleyl–NH2 over a wide range of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratios is best carried out at 45 °C. This assembly temperature provides effective control over the morphology and pore dimensions of the products. As for oleyl–NH(CH2)3NH2, the preferred assembly temperature is 60 °C. The diamine functionality provides more sites for H-bonding, thus allowing for an elevated assembly temperature and the formation of lamellar structures with better pore size fidelity.
ethanol ratios is best carried out at 45 °C. This assembly temperature provides effective control over the morphology and pore dimensions of the products. As for oleyl–NH(CH2)3NH2, the preferred assembly temperature is 60 °C. The diamine functionality provides more sites for H-bonding, thus allowing for an elevated assembly temperature and the formation of lamellar structures with better pore size fidelity.
Owing to an ability to adopt hairpin as well as extended chain structures depending on solvent polarity, oleyl amine surfactants are more versatile than saturated amines in templating both supermicroporous and mesoporous forms of silica simply by changing the polarity of the reaction medium. Saturated amines do not adopt chain conformations that allow for the formation of supermicropores. It has been suggested that supermicroporous derivatives offer greater size- and shape-selective separations and catalysis in comparison to mesoporous analogues.20 However, relatively few methods have been reported for the synthesis of supermicroporous silicas.21
Although it is possible to solvent extract and recycle amine templating agents, it is far more convenient to simply calcine the as-made mesophase to remove the surfactant. Moreover, during the lower temperature stages of the calcination process, carbon (coke) is formed in the pores of the mesophase, allowing for framework cross linking and better pore size fidelity upon the complete removal of the carbon at higher temperatures.22 The results obtained in the present study relate only to silica mesophases with spongelike and lamellar structures with pores in the supermicropore and small mesopore range. However, quaternary forms of oleyl surfactants may make it possible to template higher ordered hexagonal and cubic mesophases analogous to MCM-412, LP-MCM-4123 and MCM-4818,24 through electrostatic S+I− assembly pathways.
References
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147–1160 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Frederickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865–867 CAS.
- T. R. Pauly and T. J. Pinnavaia, Chem. Mater., 2001, 13, 987–993 CrossRef CAS.
- S. A. Bagshaw, E. Prouzet and T. J. Pinnavaia, Science, 1995, 269, 1242–1244 Search PubMed.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC.
- Y. Wang, S. Huang, S. Kang, C. Zhang and X. Li, Mater. Chem. Phys., 2012, 132, 1053–1059 CrossRef CAS.
- N. Linares, E. Serrano, M. Rico, A. Mariana Balu, E. Losada, R. Luque and J. Garcia-Martinez, Chem. Commun., 2011, 47, 9024–9035 RSC.
- Y. Horiuchi and H. Yamashita, Appl. Catal., A, 2011, 400, 1–8 CrossRef CAS.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS.
- S. Gibson, Process for the preparation of (un)saturated primary fatty amines from the corresponding fatty acids (Novagali Pharma SA, Fr.), 2007 Search PubMed Eur. Pat. Appl. EP 1746084 A1 20070124.
- A. Nagase, Y. Kuwahara, Y. Tominaga and R. Sugawara, Agric. Biol. Chem., 1983, 47, 53–58 CrossRef CAS.
- S. Sun, E. E. Fullerton, D. Weller and C. B. Murray, IEEE Trans. Magn., 2001, 37, 1239–1243 CrossRef CAS.
- B. K. H. Yen, N. E. Stott, K. F. Jensen and M. G. Bawendi, Adv. Mater., 2003, 15, 1858–1862 CrossRef CAS.
- T. R. Pauly, Y. Liu, T. J. Pinnavaia, S. J. L. Billinge and T. P. Rieker, J. Am. Chem. Soc., 1999, 121, 8835–8842 CrossRef CAS.
- A. Sayari, M. Kruk, M. Jaroniec and I. L. Moudrakovski, Adv. Mater., 1998, 10, 1376–1379 CrossRef CAS.
- R. A. Vaia, R. K. Teukolsky and E. P. Giannelis, Chem. Mater., 1994, 6, 1017–1022 CrossRef CAS.
- S. A. Bagshaw and A. R. Hayman, Adv. Mater., 2001, 13, 1011–1013 CrossRef CAS.
- Y. Zhou and M. Antonietti, Adv. Mater., 2003, 15, 1452–1455 CrossRef CAS.
- F. Kleitz, W. Schmidt and F. Schuth, Microporous Mesoporous Mater., 2003, 65, 1–29 CrossRef CAS.
- A. Sayari, P. Liu, M. Kruk and M. Jaroniec, Chem. Mater., 1997, 9, 2499–2506 CrossRef CAS.
- M. L. Pena, Q. Kan, A. Corma and F. Rey, Microporous Mesoporous Mater., 2001, 44–45, 9–16 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21023a/ |
| This journal is © The Royal Society of Chemistry 2012 |