Effective low-temperature hydrolysis of cellulose catalyzed by concentrated H3PW12O40 under microwave irradiation†
Xiutao
Li
,
Yijun
Jiang
*,
Lili
Wang
,
Lingqian
Meng
,
Wei
Wang
and
Xindong
Mu
*
Key Laboratory of Biofuels, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, Shandong 266101, P.R. China. E-mail: jiangyj@qibebt.ac.cn; muxd@qibebt.ac.cn; Fax: +86-532-80662724; Tel: +86-532-80662725
First published on 13th June 2012
Abstract
Concentrated H3PW12O40 (HPW) was first employed to decompose cellulose under microwave irradiation at low temperatures. 75.6% yield of glucose was obtained at 90 °C under microwave irradiation for 3 h, which was considerably high under such mild conditions using phosphotungstic acid as a catalyst. With the same effective acid concentration, HPW gave the highest cellulose conversion and glucose yield among the Brønsted acid catalysts, indicating that the strong Brønsted acid played an important role during cellulose hydrolysis. In the hydrolysis of cellulose with HPW as catalysts, microwave irradiation led to higher glucose yields than the conventional heating method. The recovery and reusability of HPW were investigated by extraction with diethyl ether from the reaction solution. At the same time, the performance of the concentrated HPW for real lignocellulosic biomass (corncob, corn stover and bagasse) hydrolysis was also investigated.
Introduction
Due to the increasing energy crisis and environmental concerns, finding new energy sources is becoming increasingly important.1 Biomass energy, one of the most promising alternatives to the fossil fuels is believed to have little negative impact on the environment and has attracted more and more attention.2 As is well known, glucose derived from biomass is an important platform compound which can be converted into various value-added chemicals with the fermentation or chemical processes.3–7 Therefore, the hydrolysis of cellulose into glucose is a key technology for efficient biomass utilization.Currently, several available technologies are mainly adopted for the hydrolysis of biomass. Liquid acid hydrolysis including dilute acid,8 concentrated acid9 and organic acid10,11 hydrolysis has a long industrial history, but is not recyclable and is corrosive, which leads to various environmental problems. Enzymatic hydrolysis12 is one of the most promising hydrolysis technologies, but suffers from the low hydrolysis efficiency and high cost of the enzyme. We also managed to design and synthesize sulfonated copolymer poly(acrylic acid)-co-poly(styrene sulfonic acid) to mimic the functional sites in the real enzyme, which showed high performance for the hydrolysis of polysaccharides.13 However, the separation of the catalyst is still the main problem blocking its application. Recently, solid acid catalysts such as carbonaceous solid acids,14,15 inorganic oxides,16,17 zeolites,16,18–20 cation-exchanged resins,16,17 clays16 and hetero polyacids21–23 have been reported for the hydrolysis of cellulose due to their merits of retrievability, but the limited contact between the solid acid and the cellulose greatly restricted the catalytic ability of solid acids. Generally, the hydrolysis reaction catalyzed by solid acids needs higher temperatures and gives low glucose yield. In a word, despite the success of these techniques for the hydrolysis of cellulose, each has its own pros and cons with respect to the economy, recyclability, and activity. Therefore, it is imperative to develop some novel hydrolysis technologies that can not only decompose the rigid cellulose structure effectively, like cellulase under mild temperatures, but also can be recycled like solid acids.
In this study, we describe an effective approach for the hydrolysis of cellulose at mild temperatures. In this process, the cellulose was decomposed by the concentrated HPW (H3PW12O40, 50%–88%, w/w) under microwave irradiation. It was found the concentrated HPW could convert the cellulose completely and obtain a considerably high selectivity for glucose at low temperatures (80–100 °C) under microwave irradiation. Microwave irradiation promoted the cellulose hydrolysis compared with a conventional heating method. The maximal glucose yield reached 75.6% at 90 °C under the microwave irradiation for 3 h. Notably, compared with other mineral acids (H2SO4, H3PO4), the HPW showed the highest activity for the cellulose hydrolysis with the same effective acid amount. In addition, the recovery and reusability of H3PW12O40 were also investigated by extraction with diethyl ether from the reaction solution after each run.
Experimental
The hydrolysis of cellulose was carried out in a microwave reactor (CEM, Discovers). Cellulose powder was first pretreated by ball-milling to reduce the size of the crystalline cellulose (Fig. S1†). 60 mg of milled cellulose powder and 3 mL HPW solutions were added into the reaction tube and the mixture was heated for 2 h with stirring at 100 °C. Three kinds of lignocellulosic biomass (corncob, corn stover and bagasse), successively crushed to a 40–80 mesh, were also hydrolyzed by HPW, and the hydrolysis processes were similar to those of cellulose. In a typical run, 60 mg corn stover powder was added to the reaction tube, followed by adding the concentrated HPW (3 mL). The reaction tube containing the catalyst and reactant was sealed and then placed in the CEM reactor. The mixture was stirred by a stir bar in the reactor during the reaction. After the desired reaction time, the reaction mixture was neutralized with NH3·H2O (about 0.5 mL), diluted to 50 mL with cold water, and analyzed by high-performance liquid chromatography (HPLC with RID detector, Agilent 1200 series) using a Waters sugar Pak-I column and EDTA –CaNa2 (5 mM) aqueous solution as mobile phase at 80 °C and a flow rate of 1.0 mL min−1.The conversion of cellulose was determined by measuring the soluble carbon content in the liquid product using a TOC (total organic carbon) analyzer. The yield of glucose was calculated as follows: Glucose yield (%) = amount (mol) of glucose/total amount (mol) of glucose monomer in charged polysaccharide × 100. 5-hydroxymethylfurfural (HMF) and furfural were also detected by HPLC equipped with a UV/visible detector (Waters 2489) at 284 nm using a Sun fire C18 column and methanol-H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) as the mobile phase with a flow rate of 1.0 mL min−1. HMF or furfural yield (%) = amount (mol) of HMF or furfural/total amount (mol) of sugar monomer in charged polysaccharide × 100. XRD patterns were collected on a Bruker iv8 Advance diffractometer using Cu-Kα radiation (wavelength = 1.5147 Å). The composition of the lignocellulosic biomass (Table S1,† corncob, corn stover and bagasse), was analyzed following modified NREL laboratory analytical procedures (NREL), a two-step sulfuric acid hydrolysis process was adopted to decompose the polysaccharides into sugar monomers for quantification.
4, v/v) as the mobile phase with a flow rate of 1.0 mL min−1. HMF or furfural yield (%) = amount (mol) of HMF or furfural/total amount (mol) of sugar monomer in charged polysaccharide × 100. XRD patterns were collected on a Bruker iv8 Advance diffractometer using Cu-Kα radiation (wavelength = 1.5147 Å). The composition of the lignocellulosic biomass (Table S1,† corncob, corn stover and bagasse), was analyzed following modified NREL laboratory analytical procedures (NREL), a two-step sulfuric acid hydrolysis process was adopted to decompose the polysaccharides into sugar monomers for quantification.
Results and discussion
Before the experiments, we also employed the diluted HPW solution (< 20%) to decompose the cellulose under microwave irradiation. It was found that the cellulose could only be converted at a high temperature (180 °C) with relative low glucose yield (about 56%) under optimum conditions. We believed the glucose was converted into other products by side-reactions at such a high temperature. These results agreed well with previous studies.23 In order to reduce the side-reactions and increase the glucose yield, the concentrated HPW was employed to reduce the temperature and improve the glucose yield. The boiling point of water is 100 °C: we hoped our hydrolysis method could be performed below this temperature. So in our experiments, the maximum reaction temperature was set as 100 °C. Prior to all the hydrolysis reactions, the cellulose was pretreated by ball-milling in order to decrease the crystallinity. To evaluate the effect of HPW concentration, a series of HPW solutions with different concentrations were tested for the hydrolysis of cellulose under microwave irradiation at 100 °C for 2 h. As the concentration of HPW was increased from 50% to 88%, the conversion of the cellulose was increased from 77.1% to 98.4%, as shown in Table 1. That is to say the cellulose can be almost completely converted in the 88% HPW solution at 100 °C under microwave irradiation. Meanwhile, the selectivity of the glucose was 69.2%, when the 88% HPW solution was used. However, with an increasing concentration of HPW, although the conversion of cellulose was increased greatly, the yield of glucose did not increase with the conversion of cellulose. The discrepancy between conversion and yield is thus attributed to the water-soluble polysaccharides produced during the hydrolysis of cellulose and the by-products such as 5-hydroxymethylfurfural (HMF), furfural and so on, which were also observed by others.24 Some of these by-products were determined by HPLC (Table 1, Fig. S2†) in our experiments and it was found that the yield of by-products (furfural and HMF) increased with the concentration of HPW under the microwave irradiation at 100 °C for 2h. The yield of furfural and HMF were detected to be 0.99% and 2.29% respectively, when the concentration of HPW was raised to 88%. Obviously, the high concentration of the catalyst accelerated the rate of cellulose degradation and increased the yield of glucose from cellulose under the reaction conditions at 100 °C for 2 h.| Concn. of HPW (w/w, %) | Concn. of H+ (mol L−1) | Conv. (%) | YGl (%)b | SelGl. (%) | YFu (%)c | YHM (%)c |
|---|---|---|---|---|---|---|
| a Cellulose: 60 mg; HPW solution: 3 mL; temperature: 100 °C; reaction time: 120 min. b YGl was the carbon-based yield of glucose. c YFu (%) and YHM (%) were the carbon-based yield of furfural and HMF respectively. | ||||||
| 50% | 0.82 | 77.1 | 37.1 | 48.1 | 0.42 | 0.45 |
| 60% | 1.13 | 85.2 | 54.2 | 63.7 | 0.59 | 0.69 |
| 70% | 1.53 | 87.3 | 59.3 | 67.9 | 0.64 | 0.92 |
| 80% | 2.03 | 95.1 | 65.1 | 68.5 | 0.72 | 1.14 |
| 88% | 2.63 | 98.4 | 68.1 | 69.2 | 0.99 | 2.29 |
In the following experiments, we checked the effect of the temperature on the hydrolysis of cellulose catalyzed by 88% (w/w) of HPW solution under microwave irradiation for 3 h. As shown in Fig. 1, both the conversion of the cellulose and the yield of glucose increased with increasing temperature from 80 to 90 °C, but the glucose yield drastically decreased due to the side-reactions when the temperature further went up (100 °C). Remarkably, the cellulose was selectively hydrolyzed into glucose with the glucose yield as high as 75.6% under the microwave irradiation at 90 °C for 3 h, which is a considerably high level compared to that reported so far under such mild conditions using phosphotungstic acid as catalyst. Fig. 2 shows the changes in conversion of cellulose, glucose yield and selectivity during the cellulose hydrolysis using the 88% (w/w) HPW solution as the catalyst at 100 °C. An increase in temperature resulted in an enhanced hydrolysis rate and a shortened reaction time to achieve the highest yield of glucose. 72.9% yield of glucose was obtained with the catalysis of HPW (88%, w/w) under microwave irradiation at 100 °C for 0.5 h.
 | ||
| Fig. 1 Effect of temperature on glucose yield, cellulose conversion, and glucose selectivity for cellulose hydrolysis under microwave irradiation catalyzed by HPW solution (88%, w/w). Conditions: cellulose (60 mg), HPW solution (3 mL), reaction time (3 h). | ||
 | ||
| Fig. 2 Time course of cellulose hydrolysis under microwave irradiation catalyzed by 88% (w/w) HPW solution at 100 °C. Conditions: cellulose (60 mg), HPW solution (3 mL). | ||
Fig. 3 and Fig. 4 show the plots of conversion of cellulose, glucose yield and selectivity versus time for the hydrolysis of cellulose to glucose at 90 °C catalyzed by 88% (w/w) of HPW under microwave irradiation and conventional heating (oil bath), respectively. Evidently, compared with the conventional heating method (Fig. 4), the microwave irradiation (Fig. 3) significantly accelerated the hydrolysis reaction and increased the yield of glucose. For example, the conversion of cellulose under microwave irradiation was 86.5% at 90 °C for 1 h, being almost two times higher than that under conventional heating. On the other hand, 67.6% glucose yield and 78.1% selectivity were obtained with the microwave irradiation at 90 °C for 1 h, while no glucose was obtained by using conventional heating because of the incomplete degradation of the cellulose to water-soluble polysaccharides. It was believed that the microwave irradiation might be absorbed deeply into the folding layers of the cellulose to destroy the crystal structures and improve the effective contraction between the HPW and the solid substrate, which accelerated the hydrolysis process.25 After the reaction time of 3 h under microwave irradiation at 90 °C, the yield of glucose reached the highest level, then, the attempt to prolong the irradiation time resulted in a decreased yield of glucose due to the accelerated degradation of the glucose.
 | ||
| Fig. 3 Time courses of cellulose hydrolysis under microwave irradiation catalyzed by 88% (w/w) HPW solution at 90 °C. Conditions: cellulose (60 mg), HPW solution (3 mL). | ||
 | ||
| Fig. 4 Time courses of cellulose hydrolysis under conventional heating catalyzed by 88% (w/w) HPW solution at 90 °C. Conditions: cellulose (60 mg), HPW solution (3 mL). | ||
Due to the strong Brønsted acidity approaching the super acid region, HPW exhibited better performances for both homogeneous and heterogeneous acid-catalyzed reactions than mineral acids.26,27 In order to discuss the effect of Brønsted acidity on the activity for this hydrolysis reaction, H2SO4 and H3PO4 were also employed to decompose cellulose under the same reaction conditions, which were regarded as strong and moderate acids respectively. There are general tendencies that the glucose yield and conversion decrease with an increase in the deprotonation energy (DPE), which is a probe-independent intrinsic property of an acid.28,29 The cellulose hydrolysis performance under microwave irradiation for the three acids (HPW, H2SO4 and H3PO4) are shown in Table 2. With the same effective acid concentration, both the conversion and the glucose yield after 3 h at 90 °C changed in the following order: HPW > H2SO4 > H3PO4, indicating that the strong Brønsted acid played an important role in the enhanced hydrolysis rate and glucose yield. In addition, HPW also showed the highest selectivity under the reaction condition.
There is usually a hyperbolic relationship between the rate of reaction and the quantity of substrate.30 Similarly, the effects of the amount of cellulose on the glucose yield and turn over frequency (TOF) were investigated by varying the quantity of cellulose from 0.06 g to 0.48 g. Fig. 5 shows the plot of glucose yield and TOF versus the amount of cellulose for the hydrolysis at 90 °C for 3 h under microwave irradiation. When a small quantity of cellulose was added, there was a steep increase in the TOF of the reaction with an increasing amount of cellulose, indicating that the catalytic site of the HPW was not fully utilized and the reaction rate for the hydrolysis was limited by the effective contact between the active sites of the HPW and cellulose. As the amount of cellulose increased, the catalytic sites became saturated with substrate and the glucose formation rate from cellulose depended on the activity of the HPW itself: adding more substrate would not affect the reaction rate significantly. Consequently, even though the yield of glucose decreased from 75.6% to 35.9% with the increasing amount of cellulose from 0.06 g to 0.48 g under the reaction condition for 3 h, the TOF increased from 0.035 h−1 to 0.135 h−1.
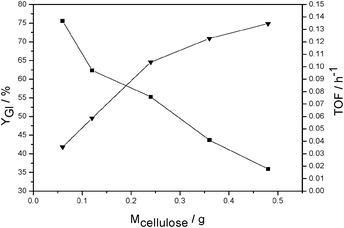 | ||
| Fig. 5 Effect of the quantity of cellulose on the glucose yield and TOF for the hydrolysis under microwave irradiation at 90 °C for 3 h catalyzed by HPW solution (88%, w/w). (■) Yield of glucose, (▲) TOF, estimated from glucose formation per mole of HPW per hour. | ||
The reusability of HPW was also investigated. After the first reaction was run at 90 °C for 3 h under microwave irradiation, the HPW was recovered from the hydrolytic solution by extraction with diethyl ether. The recovered HPW was obtained after the complete evaporation of the diethyl ether, then used for a second run under the same conditions. This recycling process was repeated six times and almost all the HPW in the solution can be recovered without considering the weight loss of the process after each run. Fig. 6 shows that the catalytic activity of HPW in the 6 cycles' reuse remained almost unchanged. Fig. 7 shows that the XRD pattern of the recovered HPW is almost the same as that of the fresh HPW. Thus it can be concluded that the structure of the HPW catalyst did not change significantly after the reaction, which can also confirm that there are no significant changes in activity for the recovered catalyst.27
 | ||
| Fig. 6 The reusability of HPW for the hydrolysis of cellulose under microwave irradiation at 90 °C for 3 h. | ||
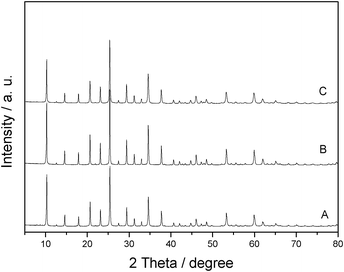 | ||
| Fig. 7 XRD patterns of the fresh (A) and the recovered H3PW12O40 after being used once (B) and seven times (C). | ||
In order to investigate the performance of the concentrated HPW for the hydrolysis of real lignocellulosic biomass, corncob, corn stover and bagasse were also employed to serve as feedstocks under microwave irradiation. Before the reaction, the composition of the lignocellulosic biomass (Table S1†) was firstly analyzed following the modified NREL laboratory analytical procedures (NREL). Table 3 gives the results for the hydrolysis of corncob, corn stover and bagasse catalyzed by 88% HPW solution at 90 °C for 3 h under microwave irradiation. For the hydrolysis of cellulose in corncob, corn stover and bagasse, 37.2%, 43.3% and 27.8% yields of glucose were obtained respectively, which were lower than that of microcrystalline cellulose (75.6%) under the same reaction conditions because of the lower reactivity of lignocellulose. It is well-established that the hydrolysis of hemicellulose (xylan + araban) takes place more readily than that of cellulose, but only 2.96%, 2.30% and 3.94% yields of xylose were obtained from the xylan in corncob, corn stover and bagasse respectively, which was attributed to the side reactions of xylose (xylose to furfural) under the same reaction conditions. The yields of the side product for furfural were detected to be 11.6%, 7.26% and 7.47% respectively. The effect of the amount of corn stover on the glucose yield was also evaluated by varying the amount of cellulose from 0.06 g to 0.5 g, as shown in Fig. S3.† With the increasing amount of corn stover from 0.06 g to 0.5 g under the same reaction conditions, the yield of glucose decreased from 43.4% to 25.6% because of the saturation of the catalytic sites, which had been discussed in Fig. 5. After hydrolysis, the reusability of HPW in corn stover hydrolysis was investigated, just as that in microcrystalline cellulose hydrolysis, and the yield of glucose gradually decreased from 31.8 (the 2nd run), 19.9 (the 3rd run) to 12.6% (the 4th run), as shown in Fig. S4.† It is believed that the by-products (furfural and HMF) and the impurities such as extractives in the corn stover (proteins, fat) generated in the hydrolysis reaction (Fig. S5†) could be extracted by the diethyl ether with HPW and damaged the catalytic site in the HPW.
| Biomass | YGl (%)b | YXy (%)b | YAr (%)b | YTs (%)b | YFu (%)c | YHM (%)c |
|---|---|---|---|---|---|---|
| a Biomass: 60 mg; HPW solution: 3 mL; temperature: 90 °C; reaction time: 180 min. b YGl (%), YXy (%), YAr (%) and YTs (%) were the carbon-based yield of glucose, xylose, arabinose and total sugar respectively. c YFu (%) and YHM (%) were the carbon-based yield of furfural and HMF respectively. | ||||||
| Corncob | 37.2 | 2.96 | 28.0 | 24.6 | 11.6 | 0.24 |
| Corn stover | 43.4 | 2.30 | 26.5 | 30.0 | 7.26 | 0.21 |
| Bagasse | 27.8 | 3.94 | 34.1 | 19.0 | 7.47 | 0.29 |
Conclusions
In summary, concentrated HPW solutions (50%–88%, w/w) exhibited excellent catalytic performance for cellulose hydrolysis under microwave irradiation at low temperatures (80–100 °C). The best result was obtained by using 88% of HPW solutions as catalyst, with a glucose yield of 75.6% for the hydrolysis of cellulose at 90 °C for 3 h under microwave irradiation. The microwave irradiation significantly accelerated the hydrolysis reaction and increased the yield of glucose as compared to the conventional heating method. After the hydrolysis of cellulose, the H3PW12O40 in the reaction mixture could be separated easily by extraction with diethyl ether, and acted as an effective catalyst for the selective hydrolysis of cellulose one more time. For the hydrolysis of real lignocellulosic biomass, HPW showed relatively lower catalytic activity than that of microcrystalline cellulose under the same reaction conditions due to the lower reactivity of lignocellulose. The reusability of HPW also can be affected in the hydrolysis of real lignocellulosic biomass.Acknowledgements
This work was supported by the Natural Science Foundation of China and Shandong Province (No. 21003146, No. 20803038, ZR2010BQ014, No. O92003110C) and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-J-10).References
- J. Pang, A. Wang, M. Zheng and T. Zhang, Chem. Commun., 2010, 46, 6935–6937 RSC.
- Y. Yu and H. Wu, Ind. Eng. Chem. Res., 2009, 48, 10682–10690 CrossRef CAS.
- N. Qureshi, T. C. Ezeji, J. Ebener, B. S. Dien, M. A. Cotta and H. P. Blaschek, Bioresour. Technol., 2008, 99, 5915–5922 CrossRef CAS.
- O. Mutschlechner, H. Swoboda and J. R. Gapes, J. Mol. Microbiol. Biotechnol., 2000, 2, 101–105 CAS.
- H. Li, W. Wang and J. F. Deng, J. Catal., 2000, 191, 257–260 CrossRef CAS.
- W. Yang and A. Sen, ChemSusChem, 2010, 3, 597–603 CrossRef CAS.
- X. Liang, C. Liu and P. Kuai, Green Chem., 2008, 10, 1318–1322 RSC.
- S. Deguchi, K. Tsujii and K. Horikoshi, Green Chem., 2008, 10, 623–626 RSC.
- E. Kontturi and T. Vuorinen, Cellulose, 2008, 16, 65–74 CrossRef.
- T. vom Stein, P. Grande, F. Sibilla, U. Commandeur, R. Fischer, W. Leitner and P. D. de Maria, Green Chem., 2010, 12, 1844–1849 RSC.
- M. Zhang, W. Qi, R. Liu, R. Su, S. M. Wu and Z. He, Biomass Bioenergy, 2010, 34, 525–532 CrossRef CAS.
- Y. H. P. Zhang, M. E. Himmel and J. R. Mielenz, Biotechnol. Adv., 2006, 24, 452–481 CrossRef CAS.
- X. Li, Y. Jiang, L. Shuai, L. Wang, L. Meng and X. Mu, J. Mater. Chem., 2012, 22, 1283–1289 RSC.
- Y. Jiang, X. Li, Q. Cao and X. Mu, J. Nanopart. Res., 2010, 13, 463–469 CrossRef.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787–12793 CrossRef CAS.
- A. Onda, T. Ochi and K. Yanagisawa, Green Chem., 2008, 10, 1033–1037 RSC.
- A. Takagaki, C. Tagusagawa and K. Domen, Chem. Commun., 2008, 5363–5365 RSC.
- P. L. Dhepe and R. Sahu, Green Chem., 2010, 12, 2153–2156 RSC.
- J. Geboers, S. V. de Vyver, K. Carpentier, P. Jacobs and B. Sels, Chem. Commun., 2011, 47, 5590–5592 RSC.
- Y. Ogaki, Y. Shinozuka, T. Hara, N. Ichikuni and S. Shimazu, Catal. Today, 2011, 164, 415–418 CrossRef CAS.
- R. Palkovits, K. Tajvidi, A. M. Ruppert and J. Procelewska, Chem. Commun., 2011, 47, 576–578 RSC.
- K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya and A. Satsuma, Green Chem., 2009, 11, 1627–1632 RSC.
- J. Tian, J. Wang, S. Zhao, C. Jiang, X. Zhang and X. Wang, Cellulose, 2010, 17, 587–594 CrossRef CAS.
- K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya and A. Satsuma, Green Chem., 2009, 11, 1627–1632 RSC.
- Y. Wu, Z. Fu, D. Yin, Q. Xu, F. Liu, C. Lu and L. Mao, Green Chem., 2010, 12, 696–700 RSC.
- A. L. Cardoso, R. Augusti and M. Da Silva, J. Am. Oil Chem. Soc., 2008, 85, 555–560 CrossRef CAS.
- W. Deng, M. Liu, Q. Zhang, X. Tan and Y. Wang, Chem. Commun., 2010, 46, 2668–2670 RSC.
- J. Macht, M. J. Janik, M. Neurock and E. Iglesia, Angew. Chem., Int. Ed., 2007, 46, 7864–7868 CrossRef CAS.
- I. A. Koppel, P. Burk, I. Koppel, I. Leito, T. Sonoda and M. Mishima, J. Am. Chem. Soc., 2000, 122, 5114–5124 CrossRef CAS.
- T. A. Bazhenova, M. A. Bazhenova, G. N. Petrova, S. A. Mironova and V. V. Strelets, Kinet. Catal., 2000, 41, 499–510 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: The composition of the lignocellulosic biomass; XRD patterns of cellulose; Chromatogram of HMF and furfural; Effect of the quantity of corn stover; The reusability of HPW for the hydrolysis of cellulose in the corn stover; HPW extracted liquid. See DOI: 10.1039/c2ra21022c/ |
| This journal is © The Royal Society of Chemistry 2012 |
