Release of encapsulated molecules from carbon nanotubes using a displacing method: a MD simulation study†
Qingzhong
Xue
*ab,
Nuannuan
Jing
b,
Liangyong
Chu
b,
Cuicui
Ling
b and
Hongxin
Zhang
a
aSchool of Electronic Engineering, Beijing University of Posts and Telecommunications, Beijing, 100876, China
bCollege of Science, China University of Petroleum, Qingdao, Shandong 266555, People's Republic of China. E-mail: xueqingzhong@tsinghua.org.cn; Fax: 86-532-86983366; Tel: 86-532-86983366
First published on 25th May 2012
Abstract
Using molecular dynamics (MD) simulations, we report a displacing method to release encapsulated molecules out of open-ended single-walled carbon nanotubes (SWNTs). The simulations indicated that encapsulated molecules such as water, DNA and indomethacin (IMT) molecules would be expelled from SWNTs by fillers such as C60 molecules and metal nanowires through a displacement process, which is attributed to the much stronger van der Waals interaction between SWNTs and fillers than that between SWNTs and encapsulated molecules. In order to quantitatively reveal the release process, the center of mass (COM) distances between encapsulated molecules (and fillers) and SWNTs were calculated. Furthermore, investigations of various energies indicated that the release process is spontaneous and the van der Waals force is the main drive for the release process. Moreover, some influencing factors such as nanotube diameter and length are discussed as well. This study provides a new method for the release of drugs/genes in the biomedical field, and provides valuable information for designing novel assembled nanoscale devices.
1. Introduction
Carbon nanotubes (CNTs) have attracted great research interest in the past 20 years not only due to their remarkable electrical,1 thermal,2 and mechanical properties,3 but also because their hollow cavities4 can serve as nanoscale templates for nanocomposites and nanostructures.5 The encapsulation of various materials, including metals, fullerenes and liquids within single-walled carbon nanotubes (SWNTs) gives encapsulated materials and SWNTs different properties and facilitates their applications. For example, metal nanoparticles enclosed in SWNTs are multishell packs composed of coaxial cylindrical shells,6 and have a lower melting point than the bulk metal,7 and significantly enhance the mechanical properties of SWNTs.8 It has been reported that C60, which is another important carbon material, can fill into SWNTs and form different ordered phases such as linear, zigzag, and helical phases inside SWNTs depending on the tube diameter.9 Using molecular dynamics (MD) simulations, Gao et al. suggested that DNA oligonucleotides could be spontaneously encapsulated inside SWNTs in a water solute environment and the van der Waals force played a dominant role.10 In addition, the transport behavior of water molecules confined in SWNTs has been reported to be different from that of a bulk system.11,12 Moreover, Hilder and Hill theoretically investigated the encapsulation behavior of drug molecules entering nanotubes in which the drug molecules are sucked in by the van der Waals force.13Within this context, how to release the encapsulated molecules out of SWNTs is another important practical issue which should be considered for applications. The possible employment of SWNTs in releasing molecules would have a wide range of applications such as novel biomedical therapies and nanopumping devices. Guest molecules would prefer to stay inside SWNTs because of the attractive interactions such as van der Waals interaction between them and the SWNTs.10 In other words, there is an energy barrier preventing encapsulated molecules inside SWNTs from running out of the hollow cavity of SWNTs. As a result, an effective driving mechanism is needed to expel the encapsulated molecules from SWNTs. Actually, transporting and releasing atoms or molecules inside SWNTs has been shown using a variety of actuation mechanisms, driven by electrical,14 optical,15 thermal16 or mechanical17,18 effects.
Several simulations of CNT-based molecule release systems have been performed by MD simulations. For instance, by extensive quantum mechanical MD simulations on an ideal model, Dai et al.14 showed that an electrically neutral fullerene ball or CNT inside an outer housing CNT can be driven to move when the housing tube is uniformly charged. Also, a laser-driven pump for atomic transport through a CNT was proposed based on the generation of electric current through the tube, which in turn would move ions in it by drag forces.15 In addition, a methodology driven by temperature for the continuous pumping of fluid through SWNTs was described by Longhurst and Quirke.16 Xue et al.19 demonstrated that the domino process can be used in SWNTs; molecules and atoms inside the SWNTs may be expelled out of the tubes when the domino wave arrives. Wang revealed that the applied torsional buckling of SWNTs has an obvious effect on the release of helium atoms18 and hydrogen molecules20 from the hollow tubes. Recently, Yu et al.21 found that water molecules confined in SWNTs can be drawn out by methane molecules, they also revealed that water molecules can transport along a system of coaxial SWNTs of different diameters with junctions under the driving force of methane molecules.22 Although a large number of research studies have focused on transportation of molecules through SWNTs, expelling encapsulated molecules from the tubes simply and effectively is still a challenging issue which has not been settled. Further studies are demanded for design of new delivery systems to release encapsulated molecules out of SWNTs.
In this paper, MD simulations have been performed to explore a new method to release encapsulated molecules out of the hollow tubes of SWNTs, which would hold great potential to be applied in the biomedical field. Because CNTs can provide an ideal environment to protect encapsulated drugs from reactions with healthy cells until the drugs are delivered to the target site, CNTs have shown great advantages to be applied as drug delivery vehicles.23–26 In addition, Bianco et al. illustrated this by highlighting a few pieces of the increasingly growing body of evidence that CNTs can be designed to be made biocompatible and biodegradable, which reinforces optimism for the future development of CNTs in biology and medicine.27 In order to promote the development of CNT-based drug/gene delivery systems, it is necessary to understand the regulation of drug/gene release and its control. However, the physical principles of the release of drug/gene molecules from CNT-based delivery systems remain unclear. Thus further studies are demanded to investigate the design of CNT-based drug/gene delivery systems. Due to the limited ability of experiments to investigate interactions between various nanoscale molecules and SWNTs, nowadays, MD simulations have emerged as an efficient way to study the transportation of molecules through SWNTs. Using MD simulations, our group has investigated the interaction between SWNTs and graphene,28 metal nanowires,29 polymers30 and biological molecules.31 This study shows that water, DNA and drug molecules can be expelled out of SWNTs by fillers such as C60 molecules and metal nanowires through a displacement process, which is attributed to the stronger van der Waals interaction between the SWNTs and the fillers. Based on this principle, we can select an appropriate replacing agent which has a strong affinity with the SWNTs to release encapsulated materials out of the cavity of SWNTs in actual applications. These observations will provide significant implications for the design of novel nanoscale devices in the biomedical field, especially in gene/drug delivery and release systems.
2. Models and computation methods
In this research, MD simulations were conducted using a commercial software package called Materials Studio.32 The interatomic interactions are described by the force field of the condensed phase optimization molecular potentials for atomistic simulation studies (COMPASS).33,34 This is the first ab initio force field that is parametrized and validated using condensed-phase properties in addition to various ab initio and empirical data and it has been widely employed in simulations for studying the interaction between CNTs and C60 fullerenes,35 water,20,36 metal nanowires,29 biomolecules31 and so on.Here, MD simulations are performed in the NVT (the volume and the temperature were constant) ensemble and periodic boundary conditions are not employed in all cases. The Andersen thermostat method is employed to control the system at room temperature (300 K). The cutoff distance for the non-bond interactions, such as the van der Waals and electronic static forces, is 9.5 Å. A time step of 1 fs is used and data was collected at intervals of 1 ps. All the simulations were performed for long enough to observe several cycles of thermal vibration. Four types of armchair SWNTs with different diameters were set up, then the water clusters, DNA segments or IMT molecules were inserted into the hollow cavities of the SWNTs. The armchair SWNTs (10, 10), (12, 12), (16, 16) and (17, 17) with a length of 49.19 Å and diameters of 13.56 Å, 16.27 Å, 21.70 Å and 23.05 Å, are fixed as rigid nanotube structures. To find the thermally stable morphology and achieve a conformation with minimum potential energy, energy minimization was performed. These minimum energy conformations were used as the initial status in the following MD simulations. The fillers, namely C60 molecules and silver nanowires were initially placed near the opening at one end of the nanotubes along the direction of the tube axis.
The MD simulations in this paper were conducted in the gas phase. Actually, in the initial work, we designed a system (SWNT–DNA–nanowire) solvated in water to run the MD simulations. The release process in the water phase can be seen in the Supporting Information.† By comparing the release process in the water phase with that in the gas phase, we find that the release processes are the same and the water solvent plays a minor role. However, the MD simulations in the water phase are quite time-consuming, and larger systems cannot run due to the limitation of computing conditions. Besides, it has been reported that the van der Waals force plays a more dominant role than the hydrophobic force in the insertion of DNA into CNTs.10 Moreover, in this paper we aim to reveal a basic principle that encapsulated molecules inside CNTs can be released by the fillers through a competitive replacement process caused by the interactions between the CNTs and molecules being different to those between the CNTs and fillers, which may be generally explored for drug release. The influence of the water soluble environment on the interactions of encapsulated molecules and fillers with CNTs is negligible. Based on the above reasons, we ran the MD simulation in the gas phase.
3. Results and discussion
3.1. Release of encapsulated molecules from SWNTs using C60 molecules displacing
 | ||
| Fig. 1 Snapshots of the release process for a water–SWNT (16, 16)–C60 system at different simulation times: (a) 0 ps, the initial structure (b) 500 ps, C60 molecules approach the SWNT (16, 16) (c) 3000 ps, water molecules are fully released, and (d) top view of final structure. | ||
In order to quantitatively explore the dynamic release process, the center of mass (COM) distance between the water molecules and the SWNT as well as that between the C60 molecules and the SWNT were plotted in Fig. 2. During the release process, the COM distance between C60 molecules and SWNT decreases rapidly and the full filling completes at about 2000 ps, as indicated by the red line, which levels off after 2000 ps. The black line, namely the COM distance between the water molecules and the SWNT, displays the opposite tendency, it is also at the time of 2000 ps that the water molecules are fully expelled out with the full encapsulation of the C60 molecules. Subsequently, the C60 molecules remain inside the cavity of the SWNT and the water molecules do not enter the SWNT again.
 | ||
| Fig. 2 COM distance between the SWNT (16, 16) and the C60 molecules and that between the SWNT (16, 16) and the water molecules during the simulation times. | ||
To reveal the physical mechanisms of the release process, we tracked different kinds of energies in the system as shown in Table 1. The total energy consists of the internal energy and the nonbond energy, while the nonbond energy consists of the van der Waals energy and the electrostatic energy. The energy of the system achieved high energy values, which depend on the large tested system including 2300 atoms. The high total energy results from the high internal energy, which consists of the valence energy and the cross-term interacting energy. The valence energy generally includes a bond stretching term, a two-bond angle term, a dihedral bond-torsion term, an inversion (or an out-of-plane interaction) term, and a Urey–Bradlay term (involving interactions between two atoms bonded to a common atom). Besides, the cross-term interacting energy accounts for the effects such as bond length and angle changes caused by the surrounding atoms and generally includes: stretch–stretch interactions between two adjacent bonds, bend–bend interactions between two valence angles associated with a common vertex atom, stretch–bend interactions between a two-bond angle and one of its bonds, stretch–torsion interactions between a dihedral angle and one of its end bonds, stretch–torsion interactions between a dihedral angle and its middle bond, bend–torsion interactions between a dihedral angle and one of its valence angles, and bend–bend–torsion interactions between a dihedral angle and its two valence angles.
| Energy (kcal mol−1) | Initial structure (E1) | Final structure (E2) | ΔE = E2 − E1 |
|---|---|---|---|
| Total energy | 41437.246034 | 39844.864914 | −1592.38112 |
| Internal energy | 44188.382958 | 44207.332400 | 18.94945 |
| Nonbond energy | −2751.136924 | −4362.467486 | −1611.330562 |
| VDW energy | 1024.1161 | −42.613495 | −1066.729595 |
| Electrostatic energy | −3775.254535 | −4319.853991 | −544.599456 |
We found that in this system, the numbers of bonds, consolidated angles, consolidated torsions, bond–bond, angle–angles and out-of-planes are 1380, 2580, 5040, 5040, 2520 and 840, respectively. As a result, the internal energy of the system achieved high energy values. However, in the release process, the energy difference between the initial configuration and the final configuration is not high. From the energy differences we found that the change of total energy is mainly caused by the change of nonbond energy, and the main contribution to the nonbond energy difference is the van der Waals energy, because the change of the van der Waals energy is largest among all the energies during the release process. Hence, it is proved that the van der Waals force is the main drive for the release process.
Furthermore, we tracked the evolution of the van der Waals interaction energy between water molecules and SWNTs and that between C60 molecules and SWNTs, as seen in Fig. 3. The van der Waals energy between C60 molecules and SWNTs decreases drastically and reaches equilibrium at around −473.03 kcal mol−1. But the van der Waals energy between water molecules and SWNTs increases slowly and reaches equilibrium at around −6.45 kcal mol−1. Besides, starting from 300 ps, when the C60 molecules began to enter into SWNTs, the van der Waals interaction energy between the C60 molecules and the SWNTs is lower than that between the water molecules and the SWNTs, which indicates much stronger van der Waals interaction between C60 molecules and SWNTs. The energy barrier would be overcome to release the water molecules because of the much stronger van der Waals interaction between the C60 molecules and the SWNTs. The deeper potential well between C60 molecules and SWNTs drives the release of water molecules. Therefore, the cavity space inside the SWNTs was occupied by the C60 molecules and the water molecules were expelled from the tubes.
 | ||
| Fig. 3 The evolution of the van der Waals energy between the water molecules and SWNT (16, 16) and that between the C60 molecules and SWNT (16, 16) during the release process. | ||
Moreover, the evolution of the potential energy of the water–C60–SWNT system is plotted in Fig. 4. The potential energy of the system shows a decreasing tendency during the initial 2000 ps, indicating the spontaneous release process. The increase of the contact area between the C60 molecules and the SWNTs reduces the systematic potential energy and enhances the stability of the whole system. Then the potential energy of the system reaches the lowest energy and begins to level off from the simulation time of 2000 ps, which indicates that the whole system reached an equilibrium state. The decrease of the potential energy of the system suggests that this release behavior is a process of energy reduction, which is in accordance with the lowest energy theory.
 | ||
| Fig. 4 The variation of potential energy as a function of simulation time for the process of water molecules released from SWNT (16, 16). | ||
Furthermore, another three types of SWNTs, (17, 17), (12, 12) and (10, 10), were also employed as the housing tubes to observe the release process. For SWNT (10, 10), the simulation was performed by introducing the C60 molecules one by one until the tube was filled up, while for SWNT (12, 12) two or three linear arrangements of C60 molecules were inserted. For SWNT (17, 17), the filling process for C60 molecules was similar to the encapsulation into SWNT (16, 16), C60 arrays can fill into the SWNT continuously because the larger number of carbon atoms in the SWNT can provide a larger attraction. The final structures are shown in Fig. 5, in which we can see that the encapsulated water molecules inside these SWNTs can all be expelled out, and the corresponding ordered phases (triple helix, zigzag and linear chain) of the C60 molecules inside the SWNTs are observed as well.
 | ||
| Fig. 5 Final structures of the water release process for different SWNTs: (a) SWNT (10, 10); (b) SWNT (12, 12); (c) SWNT (17, 17). | ||
 | ||
| Fig. 6 (a) COM distance between the SWNT and the C60 molecules and that between the SWNT (16, 16) and the DNA molecule during simulation times. (b) The potential energy as a function of simulation time in the process of DNA molecules being released from SWNT (16, 16). | ||
Three additional types of SWNTs, (17, 17), (12, 12) and (10, 10), were also employed as the housing tubes to observe the release process. Fig. 7 shows the final structures of the four designed systems when the release was completed. A notable observation is that the DNA molecule can't be completely expelled outside the SWNTs with larger diameters ((17, 17), (16,16), (12, 12)). One possible reason is that the helical and zigzag structure of the C60 molecules cannot occupy enough space inside the SWNT cavities to release teh DNA molecules completely. But for the SWNT (10, 10) system, the linear chain occupied enough space inside the cavity and continuously pushed the DNA segment out of the SWNT cavity.
 | ||
| Fig. 7 Final structures of DNA molecules released from (a) SWNT (17, 17), (b) SWNT (16, 16), (c) SWNT (12, 12), (d) SWNT (10, 10). | ||
 | ||
| Fig. 8 Final structures of IMT molecules being released from (a) SWNT (17, 17), (b) SWNT (16, 16), (c) SWNT (12, 12), (d) SWNT (10, 10). | ||
Using C60 as the replacing agent to release encapsulated molecules may not completely expel molecules with small volumes out of SWNTs with large diameters. Other fullerenes with larger diameters such as C70, C78, C90 and C120 which have also been successfully synthesised may be used as replacing agents as well. Due to their small size and strong affinity with CNTs, fullerenes can easily and flexibly fill into SWNTs and occupy the tube cavities. Therefore, fullerenes will be a promising replacing agent in the designing of nanoscale release systems.
3.2. Release of encapsulated molecules using silver nanowire displacing
A new nanoscale release system was proposed with a silver nanowire serving as the replacing agent to release encapsulated molecules such as water, DNA and IMT molecules out of SWNTs. We consider that the metal nanowire has a much larger interaction with the CNTs and the diameter and length of nanowire are controllable in the fabrication process. A nanowire with appropriate size can fill into a CNT and occupy the whole space even in the opening so that the encapsulated molecules can be released completely. Besides, as a new functional nanomaterial, silver nanoparticles have extensive applications in biomedical fields.38 Therefore, we chose silver nanowires as another replacing agent.Fig. 9 shows the process of releasing DNA molecules out of SWNT (12, 12) by the driving force of a silver nanowire. In the initial structure, the nanowire was placed along the direction of the nanotube axis and a DNA segment with three guanine bases was inserted into the cavity of the SWNT, as seen in Fig. 9(a). When the nanowire entered into tube, the DNA molecule approached the nanowire due to the attraction between them when the distance between them reached 12.3 Å, which caused the bounce back of the DNA molecule inside the SWNT, as seen in Fig. 9(b). Subsequently, the DNA molecule gradually moved to the opening of tube under the driving of the nanowire and finally got out of the tube. The final structure is shown in Fig. 9(c) (side view) and Fig. 9(d) (top view). It is noticed that the stable morphology of the cylindrical silver nanowire in the SWNT is multishell packs consisting of coaxial cylindrical shells, which is in good agreement with the structure of ultrathin copper nanowires encapsulated in CNTs.6
 | ||
| Fig. 9 Snapshots of the release process for a DNA–SWNT (12, 12)–silver nanowires system at different simulation times: (a) 0 ps, the initial structure (b) 560 ps, silver nanowires approach SWNT (12, 12) (c) 3000 ps, DNA molecules are fully released (d) top view of the final structure. | ||
As shown in Table 2, different energy changes were explored to reveal the physical mechanisms of the release process. For this system, the van der Waals energy difference between the initial structure and the final structure is −1294.047 kcal mol−1, and the total potential energy difference is −1311.686 kcal mol−1, which suggests that the change of the total potential energy is mainly caused by the change of the van der Waals energy. Furthermore, the evolution of the van der Waals interaction energy between DNA molecules and SWNTs and that between silver nanowires and SWNTs was investigated, as seen in Fig. 10. The van der Waals interaction energy between DNA molecules and SWNTs increases a little and reaches equilibrium at around −16.67 kcal mol−1 while that between silver nanowires and SWNTs decreases drastically and reaches equilibrium at around −1739.75 kcal mol−1. Besides, in the release process the van der Waals interaction energy between silver nanowires and SWNTs is much lower than that between DNA molecules and SWNTs, which indicates that there are much stronger van der Waals interactions between silver nanowires and SWNTs. Hence, the huge change of van der Waals energy between silver nanowires and SWNTs drives the release of DNA molecules and it is clear that the van der Waals interaction plays the dominant role in the release process.
 | ||
| Fig. 10 The evolution of the van der Waals energy between DNA molecules and SWNT (12, 12) and that between silver nanowires and SWNT (12, 12) during the release process. | ||
| Energy (kcal mol−1) | Initial structure (E1) | Final structure (E2) | ΔE = E2 − E1 |
|---|---|---|---|
| Total energy | −8550.005227 | −9861.6914 | −1311.686173 |
| Internal energy | 291.539961 | 273.897103 | −17.642858 |
| Nonbond energy | −8841.545188 | −10135.5885 | −1294.043315 |
| VDW energy | −8234.092037 | −9528.13949 | −1294.047453 |
| Electrostatic energy | −607.453151 | −607.449013 | 0.004138 |
Fig. 11 shows the evolution of the COM distance and the potential energy during the release process. As indicated in Fig. 11(a), the COM distance between DNA and the SWNT shows negligible change during the initial 500ps. Then as the nanowire moves close to the DNA molecule, the DNA molecule bounces back a little inside the SWNT and a decrease of the COM distance between the DNA molecule and the SWNT is observed. As shown in Fig. 11(b), the decrease of the potential energy of the system during the first 1700 ps also shows that the release process is spontaneous. The increase of the contact area between the silver nanowire and the SWNT reduces the systematic potential energy and enhances the stability of the whole system. During the simulation time from 1700 ps to 3000 ps, the COM distance and potential energy almost stay the same, which indicates that the system has reached the stable equilibrium state.
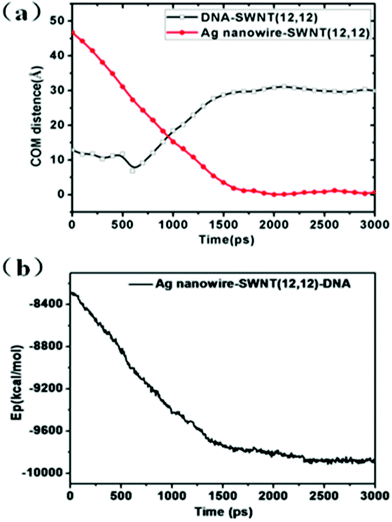 | ||
| Fig. 11 (a) COM distance between SWNT (12, 12) and silver nanowire and that between SWNT (12, 12) and DNA molecules during the simulation times. (b) The potential energy as a function of simulation time in the process of DNA molecules being released from SWNT (12, 12). | ||
In addition, simulations of the release water and drug molecules from SWNTs using silver nanowires were also performed and succeeded. Fig. 12 shows the final configuration of the release process, and it is observed that water and IMT molecules can be expelled completely outside the hollow tube of the SWNT, and the coaxial cylindrical shells of the encapsulated silver nanowire were observed as well. These simulations reveal that metal clusters such as silver nanowires can serve as replacing agents to release encapsulated molecules out of SWNTs, which will have an important application in designing nanoscale release systems.
 | ||
| Fig. 12 Final structures of (a) water molecules and (b) IMT molecules released from SWNT (12, 12). | ||
3.3. Analysis of the release process
The process of releasing encapsulated molecules out of SWNTs using C60 molecules as a replacing agent depends on the nanotube diameter size. According to several simulations, we found that C60 molecules can fill into the hollow cavity of SWNTs only when the armchair SWNT diameter is larger than a threshold value of 12.20 Å. Four types of armchair SWNT (10, 10), (12, 12), (16, 16) and (17, 17) with the same length have been selected in this study. It is found that the structures of encapsulated C60 molecules inside these hollow tubes were linear chain, zigzag, double helix and triple helix, respectively. Different structures of encapsulated C60 molecules cause different sizes of gap between the walls of the SWNT and the C60 molecules. When the gap size is big, the small molecules encapsulated will be absorbed in the inner wall of the SWNT and may not be expelled outside completely. Our simulations showed that the designed SWNT (10, 10)–C60 system was the most efficient system for releasing encapsulated molecules, even the small-sized drug molecules. The systems consisting of SWNTs with large diameters can release large-sized molecules but cannot release small-sized molecules completely. For the designed SWNT (10, 10)–nanowire system, the diameter difference between the SWNT and the nanowire should be appropriately chosen to facilitate an efficient release process. In addition to the diameter, the length of the SWNT and the fillers are also of great importance for the release efficiency. The length of the SWNTs can be designed to control the number of encapsulated molecules as well as the release efficiency. For example, multiple molecules can be encapsulated in long SWNTs and the number of these molecules released can be controlled by modulating the length of the nanowire. In order to expel all of the encapsulated molecules from cavity of the SWNT, the length of the SWNT should not be larger than that of the filler. Thus, it is important to select housing SWNTs and replacing agents with appropriate diameters and length according to different demands for designing nano-sized release systems.Conclusions
In summary, we used MD simulations to demonstrate a new displacing method to release encapsulated molecules out of SWNTs. The simulations indicated that C60 molecules and silver nanowires can serve as replacing agents to release encapsulated molecules such as water, DNA and IMT molecules out of SWNTs with different diameters. It is the van der Waals force that plays a primary role in the release processes, encapsulated molecules can be expelled out of SWNTs with the driving force of replacing agents because the van der Waals force between the SWNTs and the replacing agents is stronger than that between the SWNTs and the encapsulated molecules. The curves of the COM distance between the encapsulated molecules and the SWNTs, and those between the replacing agents and the SWNTs were plotted to quantitatively reveal the release processes. Furthermore, the decrease of the potential energy of these systems suggested that the release processes were spontaneous. Some influencing factors such as nanotube diameter and length were discussed as well. As we know, the large inner volume of SWNTs can be filled with the desired gene or drug molecules and the open ends make the inner volume accessible, molecular cargo can be successfully loaded into a nanotube by spontaneous insertion or by other external forces, thus the nanotube can used as a nanocontainer to deliver gene/drug molecules. Thus, our designed system could serve as a nanosyringe in vivo. Drug/gene molecules are sucked into the CNTs, then the nanosyringes are injected into the cell, upon release of the replacing agent (C60 molecules or nanowires), the interaction forces with the CNTs are sufficient to push the encapsulated drug/gene molecules into the cell, and then the nanosyringes are removed from cell and excreted from the body. It is expected that the nanoscale release systems will lead to further development of a broad new class of nanoscale devices in the field of biomedicine, especially in the direction of drug/gene delivery and release.Acknowledgements
This work is supported by the Program for New Century Excellent Talents in University (NCET-08-0844), the Fundamental Research Funds for the Central Universities (10CX05001A) and the Postgraduate Innovation Fund of Chain University of Pertroleum (CXZD11-16).References
- Y. S. Matthew, F. Wang, L. M. Huang, C. C. Chuang, J. Hone, S. P. O'Brien, T. F. Heinz and L. E. Brus, Science, 2004, 306, 1540–1543 CrossRef.
- R. S. Ruoff and D. C. Lorents, Carbon, 1995, 33, 925–930 CrossRef CAS.
- J. Y. Huang, S. Chen, Z. Q. Wang, K. Kempa, Y. M. Wang, S. H. Jo, G. M. Chen, S. Dresselhaus and Z. F. Ren, Nature, 2006, 439, 281–282 CrossRef CAS.
- S. C. Tsang, Y. K. Chen, P. J. F. Harris and M. L. H. Green, Nature, 1994, 372, 159–162 CrossRef CAS.
- P. M. Ajayan, O. Stephan, P. Redlich and C. C. Colliex, Nature, 1995, 375, 564–567 CrossRef CAS.
- W. Y. Choi, J. W. Kang and H. J. Hwang, Phys. Rev. B, 2003, 68, 193405 CrossRef.
- J. L. Shao, C. Yang, X. L. Zhu and X. H. Lu, J. Phys. Chem. C, 2010, 114, 2896–2902 CAS.
- L. Wang, H. W. Zhang, Z. Q. Zhang, Y. G. Zheng and J. B. Wang, Appl. Phys. Lett., 2007, 91, 051122 CrossRef.
- K. S. Troche, V. R. Coluci, S. F. Braga, D. D. Chinellato, F. Sato, S. B. Legoas, R. Rurali and D. S. Galva, Nano Lett., 2005, 5, 349–355 CrossRef CAS.
- H. J. Gao, Y. Kong, D. X. Cui and C. S. Ozkan, Nano Lett., 2003, 3, 471–473 CrossRef CAS.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188–190 CrossRef CAS.
- G. Cicero, J. C. Grossman, E. Schwegler, F. Gygi and G. Galli, J. Am. Chem. Soc., 2008, 130, 1871–1878 CrossRef CAS.
- T. A. Hilder and J. M. Hill, Nanotechnology, 2007, 18, 275704 CrossRef.
- Y. Dai, C. Tang and W. Guo, Nano Res., 2008, 1, 176–183 CrossRef CAS.
- P. Kral and D. Tomanek, Phys. Rev. Lett., 1999, 82, 5373–5376 CrossRef CAS.
- M. J. Longhurst and N. Quirke, Nano Lett., 2007, 7, 3324–3328 CrossRef CAS.
- Z. Insepov, D. Wolf and A. Hassanein, Nano Lett., 2006, 6, 1893–1895 CrossRef CAS.
- Q. Wang, Nano Lett., 2009, 9, 245–249 CrossRef CAS.
- Q. Z. Xue, D. Xia, C. Lv, N. N. Jing and C. C. Ling, J. Phys. Chem. C, 2011, 115, 20471–20480 CAS.
- Q. Wang, Carbon, 2009, 47, 1867–1885 CrossRef.
- H. Q. Yu, H. Li, J. X. Zhang, X. F. Liu and K. M. Liew, Carbon, 2010, 48, 417–423 CrossRef CAS.
- H. Q. Yu, Y. F. Li, H. Li, K. Zhang, C. G. An, X. F. Liu and K. M. Liew, J. Phys. Chem. B, 2010, 114, 8676–8679 CrossRef CAS.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 50–56 CrossRef CAS.
- R. P. Feazell, N. Nakayama-Ratchford, H. Dai and S. J. Lippard, J. Am. Chem. Soc., 2007, 129, 8438–8439 CrossRef CAS.
- S. Dhar, Z. Liu, J. Thomale, H. Dai and S. J. Lippard, J. Am. Chem. Soc., 2008, 130, 11467–11476 CrossRef CAS.
- Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Cao, X. Chen and H. Dai, Cancer Res., 2008, 68, 6652–6660 CrossRef CAS.
- A. Bianco, K. Kostarelos and M. Prato, Chem. Commun., 2011, 47, 10182–10188 RSC.
- D. Xia, Q. Z. Xue, J. Xie, H. J. Chen, C. Lv, F. Besenbacher and M. D. Dong, Small, 2010, 6, 2010–2019 CrossRef CAS.
- K. Y. Yan, Q. Z. Xue, D. Xia, H. J. Chen, J. Xie and M. D. Dong, ACS Nano, 2009, 3, 2235–2240 CrossRef CAS.
- Q. B. Zheng, Q. Z. Xue, K. Y. Yan, L. Z. Hao, Q. Li and X. L. Gao, J. Phys. Chem. C, 2007, 111, 4628–4635 CAS.
- J. Xie, Q. Z. Xue, Q. B. Zheng and H. J. Chen, Mater. Lett., 2009, 63, 319–321 CrossRef CAS.
- Materials Studio v4.4, Accelrys, Inc., San Diego, CA, USA, 2009 Search PubMed.
- H. Sun, J. Phys. Chem. B, 1998, 102, 7338–7364 CrossRef CAS.
- H. Sun, P. Ren and J. R. Fried, Comput. Theor. Polym. Sci., 1998, 8, 229–246 CrossRef CAS.
- Q. Wang, Carbon, 2009, 47, 507–512 CrossRef CAS.
- W. H. Duan and Q. Wang, ACS Nano, 2010, 4, 2338–2344 CrossRef CAS.
- L. Baweja, D. Gurbani, R. Shanker, A. K. Pandey, V. Subramanian and A. Dhawan, J. Biomed. Nanotechnol., 2011, 7, 177–178 CrossRef CAS.
- Y. Zhang and Q. B. Wang, Acta Biophysica Sinica, 2010, 26, 673–679 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figure S1: Snapshots of the release process for the DNA–SWNT (12, 12)–silver nanowire system in a water soluble environment at different simulation times. See DOI: 10.1039/c2ra20446k |
| This journal is © The Royal Society of Chemistry 2012 |
