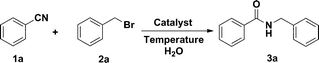
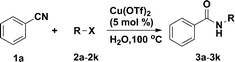
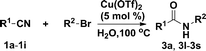
Cu(OTf)2-catalysed Ritter reaction: efficient synthesis of amides from nitriles and halohydrocarbons in water†
Gui-Rong
Qu
*a,
Yan-Wei
Song
a,
Hong-Ying
Niu
b,
Hai-Ming
Guo
*a and
John S.
Fossey
ac
aCollege of Chemistry and Environmental Science, Key Laboratory of Green Chemical Media and Reactions of Ministry of Education, Henan Normal University, Xinxiang, 453007, Henan, China. E-mail: quguir@sina.com; guohm518@hotmail.com; Fax: 86 373 3329276; Tel: 86 373 3329255
bSchool of Chemistry and Chemical Engineering, Henan Institute of Science and Technology, Xinxiang 453003, China
cSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands, B152TT, UK
First published on 13th June 2012
Abstract
An efficient and green protocol for the synthesis of amides through the Ritter reaction of nitriles and halohydrocarbons was developed. Cu(OTf)2 economically efficiently catalysed the Ritter reaction in water. A range of halohydrocarbons (benzyl, tert-butyl, sec-alkyl and primary alkyl halohydrocarbons) were coupled with nitriles, providing the corresponding amides.
Introduction
Amides are important building blocks of peptides, many natural products and synthetic materials.1 Despite numerous approaches for the formation of amides reported in the literature, the use of nitriles as starting materials remains an emerging area.1d A representative and important strategy within this field relies on the Ritter reaction, which is based on the reaction of aliphatic or aromatic nitriles and carbocations in the presence of strong acid.2 Considerable effort has been devoted to the development of catalysts (such as Lewis acids etc.) in the past decades.3 Recently, Lemaire et al.4 described the Ritter-type reaction promoted by a stoichiometric amount of copper acetate, and a green and concise methodology utilising 20 wt% sulfated tungstate as a recyclable catalyst was disclosed by Akamanchi and co-workers.3r Ritter reactions explored in the literature are mostly applied to the amidation of alcohols and alkenes.3 We are surprised to find that, to the best of our knowledge, halohydrocarbons3n,5 have rarely been utilised in the Ritter reaction. Thus, a new system utilising metal catalysts with a new starting material would be highly desirable. Herein, an efficient and general methodology was reported for the synthesis of amides via the reaction of nitriles and halohydrocarbons employing Cu(OTf)2 as a catalyst in water and the target products were achieved in satisfactory yields. Copper(II) triflate was found to be an economically efficient catalyst for the Ritter reaction, and a wide range of halohydrocarbons was employed to couple with nitriles, which expands the scope of the Ritter reaction’s starting materials.Initially, standardisation of the protocol was carried out with benzonitrile and benzyl bromide as model substrates in the presence of different catalysts at various temperatures in water under an air atmosphere. The results are shown in Table 1. First, the effect of various potential catalysts was investigated at 100 °C (entries 1–12), and Cu(OTf)2 emerged as the best choice for the present reaction. Next, the reaction was conducted under different temperature regimes confirming lower temperatures were unfavourable (entries 13–15). Increasing the amount of catalyst to 10 mol% led to no obvious improvement of the yield (entry 18). Therefore, the optimised reaction conditions were Cu(OTf)2 (5 mol%) in water at 100 °C. Under the optimised conditions, synthesis of N-benzylbenzamide was achieved in 90% yield with traces hydrolysis product.3r
|
|
|||
|---|---|---|---|
| Entry | Catalyst (mol%) | T/°C | Yieldb (%) |
| a Reaction conditions: benzonitrile (0.5 mmol), benzyl bromide (0.75 mmol), H2O (200 μL), reaction time: 5 h. b Isolated yields. | |||
| 1 | Cu(OAc)2·H2O (5) | 100 | 28 |
| 2 | CuCl2·2H2O (5) | 100 | 54 |
| 3 | CuBr2 (5) | 100 | 32 |
| 4 | CuCl (5) | 100 | 44 |
| 5 | CuBr (5) | 100 | 50 |
| 6 | CuI (5) | 100 | 60 |
| 7 | Cu(OTf)2 (5) | 100 | 90 |
| 8 | CuSO4·5H2O (5) | 100 | 20 |
| 9 | CuO (5) | 100 | 34 |
| 10 | Cu2O (5) | 100 | 50 |
| 11 | Cu (5) | 100 | trace |
| 12 | Cu(NO3)2·3H2O (5) | 100 | 32 |
| 13 | Cu(OTf)2 (5) | RT | NR |
| 14 | Cu(OTf)2 (5) | 40 | NR |
| 15 | Cu(OTf)2 (5) | 60 | 60 |
| 16 | Cu(OTf)2 (5) | 80 | 85 |
| 17 | Cu(OTf)2 (10) | 100 | 91 |
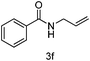
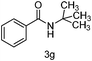
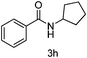
With the optimised reaction conditions in hand, the scope of nitriles and halohydrocarbons was investigated. As presented in Table 2, the substrate scope of halohydocarbons under the optimised conditions is examined. It was found that there were no remarkable electronic effects on the reaction, since benzyl halides with both electron-donating group and electron-withdrawing groups gave the corresponding products in similar yields (entries 2–5). When the same procedure was applied to allyl bromide, the target molecule was obtained in only moderate yield (entry 6). This system worked well with tert-butyl bromide, and the target compound was obtained in 90% yield (entry 7). Cyclopentyl, cyclohexyl and iso-propyl bromides could all be accommodated, and the corresponding products were delivered in satisfactory yields (87–89%) (entries 8–10). Amidation of iodoethane proceeded smoothly and lead to the target product in 80% yield (entry 11), which is rarely described in the literature.3n,5
|
|
||||
|---|---|---|---|---|
| Entry | R–X | Time/h | Product | Yieldb (%) |
| a Reaction conditions: reaction between nitriles (0.5 mmol) and halohydrocarbons (0.75 mmol) was carried out in the presence of Cu(OTf)2 (5% × 0.5 mmol) in 200 μL water at 100 °C. b Isolated yields. | ||||
| 1 | PhCH2Br | 5 |
|
90 |
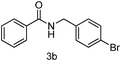
| 2 | p-BrPhCH2Br | 5 |
|
89 |
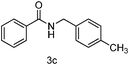
| 3 | p-CH3PhCH2Cl | 5 |
|
92 |
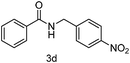
| 4 | p-NO2PhCH2Cl | 5 |
|
89 |
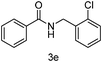
| 5 | o-ClPhCH2Cl | 5 |
|
93 |
| 6 |
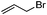
|
6 |
|
75 |
| 7 | t-C4H9Br | 6 |
|
90 |
| 8 | Cyclo-C5H9Br | 6 |
|
87 |
| 9 | cyclo-C6H11Br | 6 |
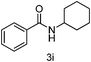
|
89 |
| 10 | iso-propylBr | 6 |

|
88 |
| 11 | CH3CH2I | 7 |
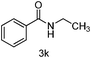
|
80 |
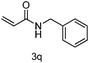
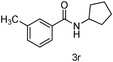
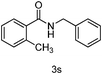
Next the effect of nitriles was also investigated, as outlined in Table 3. The results indicate that electron-donating and electron-withdrawing groups on the para site of the benzonitrile may be accommodated (entries 2 and 3). Similar results were obtained for the meta substituted benzonitriles (entries 4, 5 and 8). Ortho tolyl benzonitrile gave rise to the desired product in 91% yield (entry 9). Unfortunately, when the system was applied to 2,6-dichlorobenzonitrile no reaction was observed (entry 6). In order to further demonstrate the scope of this system, acrylonitrile was also used affording the corresponding target product in 80% yield (entry 7).
|
|
||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yieldb (%) |
| a Reaction conditions: reaction between nitriles (0.5 mmol) and halohydrocarbons (0.75 mmol) was carried out in the presence of Cu(OTf)2 (5% × 0.5 mmol) at 100 °C for 5–8 h. b Isolated yields. | ||||
| 1 | Ph | PhCH2 |
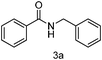
|
90 |
| 2 | p-CH3OPh | PhCH2 |
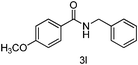
|
89 |
| 3 | p-NO2Ph | PhCH2 |
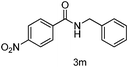
|
89 |
| 4 | m-NO2Ph | cyclo-C5H9 |
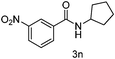
|
90 |
| 5 | m-CH3Ph | PhCH2 |
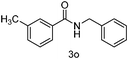
|
90 |
| 6 | 2,6-Cl2Ph | PhCH2 |
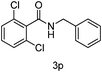
|
NR |
| 7 | ethenyl | PhCH2 |
|
80 |
| 8 | m-CH3Ph | cyclo-C5H9 |
|
90 |
| 9 | o-CH3Ph | PhCH2 |
|
91 |
In conclusion, we have developed an efficient and general protocol for the synthesis of amides via the reaction of nitriles and halohydrocarbons employing Cu(OTf)2 as catalyst in water. Cu(OTf)2 was shown to be an economically efficient catalyst for the Ritter reaction. Amidation of a series of halohydrocarbons such as benzyl, tert-butyl, sec-alkyl and primary alkyl halohydrocarbons proceeded smoothly to afford the corresponding products in satisfactory yields, which expanded the scope of the Ritter reaction by using halohydrocarbons as a starting material.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Grant Nos 21072047, and 21172059), the Program for New Century Excellent Talents in University of Ministry of Education (No. NCET-09-0122), Excellent Youth Foundation of Henan Scientific Committee (No. 114100510012), the Program for Changjiang Scholars and Innovative Research Team in University (IRT1061), the Program for Innovative Research Team in University of Henan Province (2012IRTSTHN006), and the Excellent Youth Program of Henan Normal University. JSF thanks Henan Normal University for a visiting professorship and the University of Birmingham for support.References
- (a) G. S. Singh, Tetrahedron, 2003, 59, 7631 CrossRef CAS; (b) F. Albericio, Curr. Opin. Chem. Biol., 2004, 8, 211 CrossRef CAS; (c) B. Shen, D. M. Makley and J. N. Johnston, Nature, 2010, 465, 1027 CrossRef CAS; (d) C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405 RSC.
- (a) J. J. Ritter and P. P. Minieri, J. Am. Chem. Soc., 1948, 70, 4045 CrossRef CAS; (b) J. J. Ritter and J. Kalish, J. Am. Chem. Soc., 1948, 70, 4048 CrossRef CAS; (c) L. I. Krimen and D. L. Cota, Org. React., 1969, 17, 213 CAS; (d) D. H. R. Barton, P. D. Magnus and R. N. Young, J. Chem. Soc., Chem. Commun., 1973, 331 RSC; (e) D. H. R. Barton, P. D. Magnus, J. A. Garbarino and R. N. Young, J. Chem. Soc., Perkin Trans. 1, 1974, 2101 RSC; (f) S. Top and G. Jaouen, J. Org. Chem., 1981, 46, 78 CrossRef CAS; (g) A. Garcia Martinez, R. Martinez Alvarez, E. Teso Vilar, A. Garcia Fraile, M. Hanack and L. R. Subramanian, Tetrahedron Lett., 1989, 30, 581 CrossRef CAS; (h) M. Mukhopadhyay and J. Iqbal, J. Org. Chem., 1997, 62, 1843 CrossRef CAS; (i) M. Y. Lebedev and M. B. Erman, Tetrahedron Lett., 2002, 43, 1397 CrossRef CAS; (j) K. L. Reddy, Tetrahedron Lett., 2003, 44, 1453 CrossRef CAS; (k) J. Liu, Ch. F. Ni, Y. Li, L. J. Zhang, G. Y. Wang and J. B. Hu, Tetrahedron Lett., 2006, 47, 6753 CrossRef CAS.
- (a) P. Salehi and A. R. Motlagh, Synth. Commun., 2000, 30, 671 CrossRef CAS; (b) M. M. Lakouraj, B. Movassagh and J. Fasihi, Synth. Commun., 2000, 30, 821 CrossRef CAS; (c) R. M. A. Pinto, J. A. R. Salvador and C. Le Roux, Synlett, 2006, 30, 2047 Search PubMed; (d) V. R. Kartashov, K. V. Malkova, A. V. Arkhipova and T. N. Sokolova, Russ. J. Org. Chem., 2006, 42, 966 CrossRef CAS; (e) E. Callens, A. J. Burtonb and A. G. M. Barrett, Tetrahedron Lett., 2006, 47, 8699 CrossRef CAS; (f) R. Sanz, A. Martinez, V. Guilarte, J. M. Alvarez-Gutierrez and F. Rodriguez, Eur. J. Org. Chem., 2007, 4642 CrossRef CAS; (g) V. Polshettiwar and R. S. Varma, Tetrahedron Lett., 2008, 49, 2661 CrossRef CAS; (h) J. S. Yadav, B. V. S. Reddy, T. Pandurangam, Y. J. Reddy and M. K. Gupta, Catal. Commun., 2008, 9, 1297 CrossRef CAS; (i) M. Barbero, S. Bazzi, S. Cadamuro and S. Dughera, Eur. J. Org. Chem., 2009, 430 CrossRef CAS; (j) B. Anxionnat, A. Guérinot, S. Reymond and J. Cossy, Tetrahedron Lett., 2009, 50, 3470 CrossRef CAS; (k) L. Ma'mani, A. Heydari and M. Sheykhan, Appl. Catal., A, 2010, 384, 122 CrossRef CAS; (l) P. Theerthagiri, A. Lalitha and P. N. Arunachalam, Tetrahedron Lett., 2010, 51, 2813 CrossRef CAS; (m) B. V. S. Reddy, N. S. Reddy, C. Madan and J. S. Yadav, Tetrahedron Lett., 2010, 51, 4827 CrossRef; (n) R. G. Kalkhambkar, S. N. Waters and K. K. Laali, Tetrahedron Lett., 2011, 52, 867 CrossRef CAS; (o) F. Tamaddon and F. Tavakoli, J. Mol. Catal. A: Chem., 2011, 337, 52 CrossRef CAS; (p) R. I. Khusnutdinov, N. A. Shchadneva, Yu. Yu. Mayakova, L. F. Khisamova and U. M. Dzhemilev, Russ. J. Org. Chem., 2011, 47, 1682 CrossRef CAS; (q) C. Cazorla, E. Métay and M. Lemaire, Tetrahedron, 2011, 67, 8615 CrossRef CAS; (r) K. V. Katkar, P. S. Chaudhari and K. G. Akamanchi, Green Chem., 2011, 13, 835 RSC; (s) H. Firouzabadi, N. Iranpoor and A. Khoshnood, Catal. Commun., 2008, 9, 529 CrossRef CAS.
- C. Cazorla, E. Métay, B. Andrioletti and M. Lemaire, Tetrahedron Lett., 2009, 50, 6855 CrossRef CAS.
- N-M. Bi, M-G. Ren and Q-H. Song, Synth. Commun., 2010, 40, 2617 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: General information, experimental procedures, and characterization data for the products including spectroscopic information. See DOI: 10.1039/c2ra20941a/ |
| This journal is © The Royal Society of Chemistry 2012 |