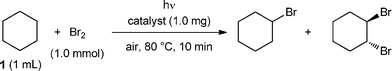
Biogenic manganese oxide: effective new catalyst for direct bromination of hydrocarbons†
Yuta
Nishina
*a,
Hideki
Hashimoto
*b,
Noriyasu
Kimura
b,
Naoyuki
Miyata
c,
Tatsuo
Fujii
b,
Bunsho
Ohtani
d and
Jun
Takada
b
aResearch Core for Interdisciplinary Sciences, Okayama University, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan. E-mail: nisina-y@cc.okayama-u.ac.jp; Fax: (+81) 86-251-8718; Tel: (+81) 86-251-8718
bGraduate School of Natural Science and Technology, Okayama University, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan
cDepartment of Biological Environment, Akita Prefectural University, Akita 010-0195, Japan
dCatalysis Research Center, Hokkaido University, Sapporo 001-0021, Japan
First published on 4th July 2012
Abstract
Nano- and micro-architectural manganese oxide produced by microorganisms catalyses the selective monobromination of hydrocarbons in excellent yield under irradiation of fluorescent room light. The knowledge gained from investigating natural materials will lead to unprecedented catalyst designing toward efficient organic reactions.
Recently, eco- and cost-effective synthetic methods of high performance catalysts have been sought-after, with increasing demands for energy-saving and inexpensive ways in various fields of science. In most cases, catalysts have required precious metal components (e.g., Pt, Pd, Au, Rh, etc.) and abundant energy for purification, preparation, and modification of catalyst surfaces. Here we propose to use biogenic manganese oxide (BMO) produced by microorganisms under environmentally benign conditions (at ordinary temperature and pressure, and neutral pH)1–3 as catalysts for important chemical reactions. Although many geochemical and microbiological perspectives on BMO have been published,4,5 and some potential applications have been proposed,6,7 there are no reports on using BMO as a catalyst of practical organic reactions. Considering its low-crystalline feature and large surface area,8–11 we expect BMO to work as an effective catalyst.
In a chemical reaction screening, we found that BMO can catalyse the reaction of hydrocarbons and Br2. The products, organobromides, are important starting materials and intermediates in organic syntheses and in the fields of petroleum, pharmaceuticals and agricultural chemistry.12–16 Currently, the synthesis of organobromides involves significant energy- and resource-consuming multistep (4-step) preparation via alcohols and HBr or PBr3.17,18 The best method to obtain organobromides is one-step catalytic synthesis from hydrocarbons and Br2. However, although a number of studies have reported the direct bromination of hydrocarbons using heat, light,19 additives (excess AcOH,20 stoichiometric tert-BuONa21 and excess MnO222) and catalysts (AlBr3),23 no practical method has yet been discovered. In this study, we successfully established the direct and selective bromination of hydrocarbons using catalytic amounts of BMO under short reaction times and easily accessible temperatures with fluorescent room light.
BMO was obtained from enrichment cultures of Mn-oxidizing microorganisms (see ESI†).24,25 Morphological observation by scanning electron microscopy (SEM) shows that BMO is a mixture of amorphously agglomerated structures (Fig. 1a, moiety other than arrows), hollow globules (white arrow and Fig. 1b) and small globules (red arrows and Fig. 1c). This morphology of hollow and small globules is similar to those of Siderocapsa spp.26 and Acremonium sp.27 Although the presence of such a variety of morphotypes indicates that diverse microorganisms are involved in BMO production, almost all structural motifs have the same erose surface as determined by SEM (Fig. 1a–c) and scanning transmission electron microscopy (STEM, Fig. 1d) and contain the same primary structural units.
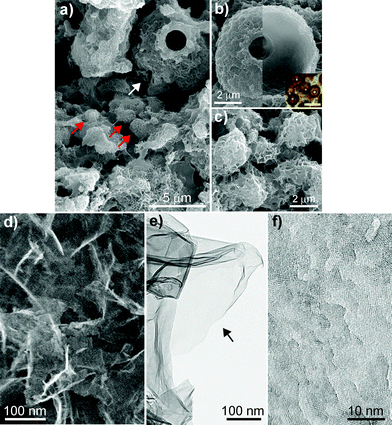 | ||
| Fig. 1 Micrographs of BMO. a) Typical SEM image of BMO. b) SEM image of a hollow globule with a rough outer surface (left) and inner globule (right). Inset image is observed using light microscopy. Bar, 10 μm. c) SEM image of small globules. d) STEM image of the rough surface using secondary electron detector. e) TEM image of a BMO nanosheet. Arrow indicates a single nanosheet. f) TEM image of the surface of a BMO nanosheet. | ||
The primary structural units of BMO, as determined by transmission electron microscopy (TEM) and STEM, are 1.6-nm-thick nanosheets (Fig. 1e, arrow) intertwined complexly to form a porous structure with irregular open channels (Fig. 1d). The erose appearance of the surface of all structures (Fig. 1d) is attributed to edge moieties and surface folding; the nanosheet surface shows a terrace structure with many steps (Fig. 1f). Because of these nanostructural features, BMO has a large surface area (130 m2 g−1, see Fig. S1 in the ESI†). Crystal structure analyses (see Fig. S2 and S3 in the ESI†) show that the structure of BMO is similar to that of birnessite (e.g., Na0.31MnO1.91·0.7H2O), which has a layer structure of edge-sharing MnO6 octahedral sheets with interlayer cations (e.g., Na+) and water molecules (see Fig. S2 in the ESI†) .28
BMO's chemical composition, determined by energy-dispersive X-ray spectroscopy (EDX), is roughly Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca
Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl
Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 83
Al = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in at% (H, C and O excepted). The main elements (Mn, Ca, Cl, P and Mg) are homogeneously distributed, as determined by STEM-EDX (see Fig. S4 in the ESI†), suggesting that the interlayer cations may be Ca2+ and/or Mg2+. The mean Mn oxidation state, as determined by X-ray absorption fine-structure spectroscopy (XAFS) (see Fig. S5 in the ESI†), is Mn3.3+, which indicates a mixture of the oxidation states of Mn2+, Mn3+ and Mn4+. These porous features, nanostructure and mixed oxidation states of BMO are extremely attractive characters for catalysis.
1 in at% (H, C and O excepted). The main elements (Mn, Ca, Cl, P and Mg) are homogeneously distributed, as determined by STEM-EDX (see Fig. S4 in the ESI†), suggesting that the interlayer cations may be Ca2+ and/or Mg2+. The mean Mn oxidation state, as determined by X-ray absorption fine-structure spectroscopy (XAFS) (see Fig. S5 in the ESI†), is Mn3.3+, which indicates a mixture of the oxidation states of Mn2+, Mn3+ and Mn4+. These porous features, nanostructure and mixed oxidation states of BMO are extremely attractive characters for catalysis.
We designed one-step synthesis of organobromides and examined the effectiveness of BMO as a catalyst. Surprisingly, bromocyclohexane (1′) was obtained selectively in 91% yield (0.91 mmol) (Table 1, entry 1). Moreover, we investigated the bromination of 1 without BMO. Product 1′ was obtained but the yield and selectivity were low to give polybrominated products (Table 1, entry 3), suggesting that the BMO surface provides reactive sites, and facilitates highly selective monobromination.
| Entry | Catalyst | Yield of 1′/% | Yield of 1′′/% |
|---|---|---|---|
| a All reactions were performed using 1 mL of substrate, 0.052 mL (1.0 mmol) of Br2 and 1.0 mg of BMO in a sealed tube. The product yield was determined based on the mole of Br2 by GC using dodecane as internal standard. b The reaction was performed in the absence of light. | |||
| 1 | BMO | 91 | < 1 |
| 2b | BMO | 28 | 1 |
| 3 | None | 13 | 8 |
| 4b | None | 1 | 0 |
| 5 | MnO2 (115 m2 g−1) | 26 | 1 |
| 6 | MnO2 (Ca, Mg, etc.) | 21 | 1 |
| 7 | Mn2O3 | 42 | 2 |
| 8 | MnO | 52 | 4 |
| 9b | MnO | 15 | 2 |
| 10 | Na-birnessite | 55 | 5 |
| 11b | Na-birnessite | 2 | 0 |
We found that the effect of light was also important. The yield of 1′ decreased from 91% in the presence of light to 28% in the absence of light (Table 1, entry 2). Continuous irradiation is necessary, because the yield of 1′ decreased to 69% when irradiation was performed only for the first 5 min. The best light source was ambient fluorescent room light. In contrast, irradiation with strong light sources such as a high pressure mercury-vapour lamp (Ushio, UM-452, 450 W) or a xenon lamp (Asahi Spectra, MAX-302, 300 W) gave complex polybrominated products derived from the photoinduced formation of free bromine radicals.
Next, we investigated the wavelength dependence of cyclohexane (1) bromination (Fig. 2). Bromine absorbed light at 380–500 nm (Fig. 2, –) and this tendency was similarly observed in the action spectrum without BMO (Fig. 2, ○). The action spectrum with BMO has a peak top at 335 nm (Fig. 2, ●) that shifts wavelength shorter than those of former two spectra, indicating that the reaction with BMO proceeds efficiently in the ultraviolet region. Stoimenov et al. reported that when Br2 is adsorbed on the surface of magnesium oxides, its photoabsorption spectrum shifted to a wavelength shorter than that of free Br2.29 The aforementioned results and background led us to suggest that the shorter wavelength shift of the action spectrum with BMO could indicate that BMO and Br2 share some types of chemical and/or physical bonds on the BMO surface, and that BMO and/or Br2 adsorbed on BMO functions as a photoabsorber. Additionally, the fact that the apparent quantum efficiency with BMO exceeds unity (1.0) suggests that photoinduced bromination involves a radical chain reaction.
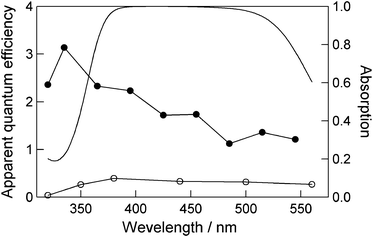 | ||
| Fig. 2 Action spectra of photoinduced bromination of cyclohexane in the presence (●) and absence (○) of BMO (left axis); photoabsorption spectrum of Br2 (–) in cyclohexane (right axis). | ||
For comparison, we investigated the bromination of 1 under two alternate sets of conditions. First, we performed bromination using previously reported reagents and catalysts; none of them outperformed BMO (see Fig. S6 in the ESI†).18–22 Second, we performed bromination using other types of manganese oxides (for preparation details, see methods in the ESI†); this clarified the reason for the high catalyst activity of BMO. Two Mn4+O2, one with the surface area of 115 m2 g−1 (Table 1, entry 5) and the other with a chemical composition similar to that of BMO (Table 1, entry 6),30 gave 1′, but only in 26 and 21% yield, respectively. Similarly, Mn3+2O3 (Table 1, entry 7)30 also performed poorly. In contrast, Mn2+O (Table 1, entry 8)30 and Na-birnessite (Mn3.5+, as determined by XAFS) (Table 1, entry 9),30 which has a crystal structure similar to that of BMO, gave 1′ in relatively high yield of 52 and 55%, respectively.
These results suggest that two properties of BMO are particularly important for determining its catalytic activity: (1) a valence state of Mn3.3+ (a mixed state containing Mn2+, Mn3+ and Mn4+, particularly Mn2+), and (2) a layered crystal structure with MnO6 octahedral sheets. Additionally, BMO's intertwining nanosheets (Fig. 1d) should make its structure porous enough to facilitate the approach of reactants; its erose surface and numerous edge moieties (Fig. 1f) should provide effective reactive sites and also enhance reactivity and selectivity.
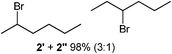
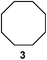
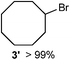
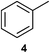
We investigated various bromination substrates listed in Table 2. Alkyl moieties, e.g., hexane (a linear alkane, Table 2, entry 1), cyclooctane (a large cyclic alkane, Table 2, entry 2) and benzyl position of toluene (Table 2, entry 3), are readily monobrominated with high yield under mild conditions. The second bromine can be introduced with the decrease in product yield when 1′ was subjected to similar reaction conditions (Table 2, entry 4), supporting the selective monobromination (Table 1, entry 1). In contrast, aromatic moieties are monobrominated only under intense conditions (Table 2, entries 5 and 6). These tendencies suggest that BMO can promote bromination by two different mechanisms—a radical pathway for an alkyl moiety and electrophilic addition to cationic bromine for an aromatic moiety—because the dissociation energy of an aromatic C–H bond is higher than that of a primary alkyl moiety. Halogens could interact with metal oxides both with and without polarization.29 If this were true, BMO would promote both radical- and cationic-derived bromination. We observed the following order of C–H bond reactivity for hydrocarbons: benzylic (Table 2, entry 3) > secondary alkyl (Table 1, entry 1; Table 2, entries 1, 2, and 4) > aromatic (Table 2, entries 5 and 6) > primary alkyl (Table 2, entries 1 and 6).
| entry | substrate | conditions | product |
|---|---|---|---|
| a All reactions were performed using 1 mL of substrate, 0.052 mL (1.0 mmol) of Br2 and 1.0 mg of BMO under ambient fluorescent room light at the designated reaction conditions. The product yield was determined after isolation with distillation based on the mole of Br2. | |||
| 1 |
|
50 °C, 10 min |
|
| 2 |
|
80 °C, 20 min |
 σ σ |
| 3 |
|
25 °C, 10 min |
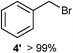
|
| 4 |
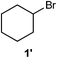
|
80 °C, 20 min |

|
| 5 |
|
100 °C, 24 h |
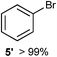
|
| 6 |
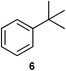
|
100 °C, 24 h |
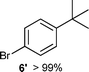
|
In summary, we adopted a unique approach toward catalyst exploration and found that low-cost, eco-friendly and ubiquitous BMO exhibits high catalytic activity for the photoinduced direct bromination of hydrocarbons. The catalytic activity of BMO exceeds that of artificially synthesized Mn oxides because of the specific nanostructure and oxidation state of BMO. Our future work includes investigation of interdisciplinary synthetic methodologies involving both biological and artificial processes with the goal of continuing to develop important organic reactions that are beyond the reach of current catalyst investigations.
Acknowledgements
Synchrotron radiation experiments were performed at BL9C in the Photon Factory with the approval of the Photon Factory Program Advisory Committee (Proposal No. 2009G024). This study was financially supported by the Special Funds for Education and Research from the Ministry of Education, Culture, Sports, Science and Technology (J.T.), Funds for the Development of Human Resources in Science and Technology (Y.N.), Hatakeyama Culture Fundation (Y.N.) and Okayama Foundation for Science and Technology (H.H. and Y.N.). This study was also supported by the Cooperative Research Program of Catalysis Research Center, Hokkaido University. (Grant #11B2001).References
- W. C. Ghiorse, Annu. Rev. Microbiol., 1984, 38, 515 CrossRef CAS.
- B. M. Tebo, et al. , Annu. Rev. Earth Planet. Sci., 2004, 32, 287 CrossRef CAS.
- G. M. Gadd, Microbiology, 2010, 156, 609 CrossRef CAS.
- T. Hennebel, B. De Gusseme, N. Boon and W. Verstraete, Trends Biotechnol., 2009, 27, 90 CrossRef CAS.
- N. Miyata, Y. Tani, M. Sakata and K. Iwahori, J. Biosci. Bioeng., 2007, 104, 1 CrossRef CAS.
- M. Villalobos, J. Bargar and G. Sposito, Environ. Sci. Technol., 2005, 39, 569 CrossRef CAS.
- S. Grangeon, B. Lanson, N. Miyata, Y. Tani and A. Manceau, Am. Mineral., 2010, 95, 1608 CrossRef CAS.
- V. Petkov, Y. Ren, I. Saratovsky, P. Pasten, S. J. Gurr, M. A. Hayward, K. R. Poeppelmeier and J. F. Gaillard, ACS Nano, 2009, 3, 441 CrossRef CAS.
- Y. M. Nelson, L. W. Lion, W. C. Ghiorse and M. L. Shuler, Appl. Environ. Microbiol., 1999, 65, 175 CAS.
- M. Villalobos, J. Bargar and G. Sposito, Environ. Sci. Technol., 2005, 39, 569 CrossRef CAS.
- G. Rosseels, C. Houben, P. Kerckx, in Advances in Organobromine Chemistry II (ed.: J. R. Desmurs, B. Gérard, M. J. Goldstein), Elsevier, Amsterdam, 1995, 152–225 Search PubMed.
- H. J. Cristau, J. R. Desmurs, in Advances in Organobromine Chemistry II (ed.: J. R. Desmurs, B. Gérard, M. J. Goldstein), Elsevier, Amsterdam, 1995, 240–302 Search PubMed.
- P. E. Rakita, G. S. Silverman, F. Sato, H. Urabe, in Handbook of Grignard Reagents (ed.: G. S. Silverman, P. E. Rakita), Marcel Dekker, New York, 1996, 1 Search PubMed.
- A. M. Echavarren, D. J. Cárdenas, in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004, 1–49 Search PubMed.
- P. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis and V. A. Vu, Angew. Chem., Int. Ed., 2003, 42, 4302 CrossRef CAS.
- G. W. Gribble, Chem. Soc. Rev., 1999, 28, 335 RSC.
- O. Kamm, C. S. Marvel, R. H. Goshorn, T. Boyd and E. F. Degering, Org. Synth., 1921, 1, 3 Search PubMed.
- L. H. Smith, Org. Synth., 1943, 23, 88 CAS.
- G. Egloff, R. E. Schaad and C. D. Lowry, Chem. Rev., 1931, 8, 1 CrossRef CAS.
- T. M. Shaikh and A. Sudalai, Tetrahedron Lett., 2005, 46, 5589 CrossRef CAS.
- R. Montoro and T. Wirth, Synthesis-Stuttgart, 2005, 1473 CAS.
- X. F. Jiang, M. H. Shen, Y. Tang and C. Z. Li, Tetrahedron Lett., 2005, 46, 487 CrossRef CAS.
- I. S. Akhrem, A. V. Orlinkov, L. V. Afanaseva, E. I. Mysov and M. E. Volpin, Tetrahedron Lett., 1995, 36, 9365 CrossRef CAS.
- Y. Tani, N. Miyata, K. Iwahori, M. Soma, S. Tokuda, H. Seyama and B. K. G. Theng, Appl. Geochem., 2003, 18, 1541 CrossRef CAS.
- N. Miyata, Y. Tani, M. Sakata and K. Iwahori, J. Biosci. Bioeng., 2007, 104, 1 CrossRef CAS.
- H. H. Hanert, in The Prokaryotes, ed. M. Dworkin, Springer, 2006, 1005 Search PubMed.
- N. Miyata, Y. Tani, K. Maruo, H. Tsuno, M. Sakata and K. Iwahori, Appl. Environ. Microbiol., 2006, 72, 6467 CrossRef CAS.
- Q. Feng, E. H. Sun, K. Yanagisawa and N. Yamasaki, J. Ceram. Soc. Jpn., 1997, 105, 564 CrossRef CAS.
- P. K. Stoimenov, V. Zaikovski and K. J. Klabunde, J. Am. Chem. Soc., 2003, 125, 12907 CrossRef CAS.
- Surface area of MnO2 (Ca, Mg, etc.), Mn2O3, MnO, and Na-birnessite was estimated to be 14, 3, 0.6, and 12 m2 g−1, respectively.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, characterization data and action spectra preparation. See DOI: 10.1039/c2ra20896b/ |
| This journal is © The Royal Society of Chemistry 2012 |