Direct synthesis of amine-functionalized MIL-101(Cr) nanoparticles and application for CO2 capture†
Yichao
Lin
,
Chunlong
Kong
* and
Liang
Chen
*
Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, Zhejiang 315201, China. E-mail: kongchl@nimte.ac.cn; chenliang@nimte.ac.cn
First published on 13th June 2012
Abstract
A pure amine-functionalized MIL-101(Cr) has been synthesized for the first time by a simple method. The as-prepared nanoparticles are around 50 nm. In addition, the resulting amine-functionalized MIL-101(Cr) displayed excellent CO2 adsorption capacity, up to 15 mmol g−1 at 16 °C.
Metal–organic frameworks (MOFs) have emerged as an intriguing class of porous materials due to their striking physical and chemical properties.1 They have shown promising applications in catalysis, drug delivery and sensing or recognition by virtue of their high surface area, high thermal stability and permanent porosity.2 Furthermore, owing to their high surface area and distinct porosity, MOFs are good candidates for gas storage and separation.3 Recently, the synthesis of MOFs with functional groups has received considerable attention since the functional groups can display desirable properties and allow post-synthetic modification.4 For example, the capacity for gas sorption in MOFs can be improved by chemical functionalization of the pores with functional groups that interact strongly with the adsorbates.5 However, the synthesis of functionalized MOFs is very challenging because many chemical functionalities are incompatible with the conditions for MOF assembly, and thus cannot be obtained via traditional synthetic routes.6 In this regard, the development of new functionalized MOFs via a simple and efficient synthesis method is highly demanded.7
On the other hand, CO2 capture is one of the most important solutions to the increasingly severe global climate problem. It is well known that amine-functionalized MOFs exhibit promise for application in CO2 capture arising from the combination of amino groups and MOFs structure. Developing new functionalized MOFs for CO2 capture and separation is critically important.8 We report the direct synthesis of amine-functionalized MIL-101(Cr), which was obtained by the reaction of chromic nitrate and 2-aminoterephthalic acid (ATPA) using OH−-assisted hydrothermal synthesis at 150 °C for an optimized reaction time (12 h) (Fig. 1a). Moreover, the average particle size was around 50 nm, as estimated by SEM and TEM (Fig. 1b and 1c). In contrast, our initial attempts to fabricate such a MOF by means of the hydrothermal reaction of chromic nitrate and ATPA in aqueous or sodium chloride added aqueous solution resulted in the precipitation of unreacted starting materials (Fig. S1 in the Supporting Information†). Herein we propose a mechanism to explain the formation of this nanoscale amine-functionalized MOF (Scheme 1). It is well known that OH− ions would promote the dissolution of the organic acid (e.g., ATPA) in aqueous solution because ATPA2− ions were generated (Fig. S2 in the Supporting Information†). The high concentration of ATPA2− accelerates the formation of crystal nuclei. As a result, the high concentration of “MOF nuclei” would yield small crystal particles after further reaction. Similarly, it has been reported that high pH can decrease the resulting MOF particle size.9 Herein the results further suggest that high pH can facilitate the formation of nanoscale functionalized MOFs. This highlights the role of pH in the synthesis of new functionalized MOFs. It should be noted that few CrO2− ions could be present in the synthesis solution (Table S1 in the Supporting Information†). In addition, we note that there are few reports on the synthesis of nanoscale MOFs.10,11
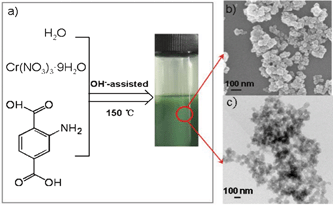 | ||
| Fig. 1 (a) Synthesis of amine-functionalized MIL-101(Cr) using an OH−-assisted hydrothermal technique. (b) SEM image of the as-prepared particles. (c) TEM image of the as-prepared particles. | ||
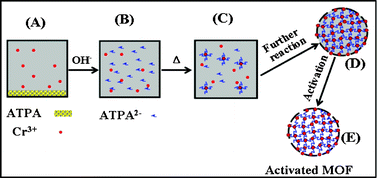 | ||
| Scheme 1 Schematic illustration of the formation of nanoscale amine-functionalized MIL-101(Cr). | ||
The result of powder X-ray diffraction (PXRD) indicates that the resulting nanoscale MOF was a typical MIL-101(Cr) structure (Fig. 2a).1a The rather broad Bragg reflections were due to the fact that the resulting product consisted of small nanoparticles (∼50 nm), which was similar to that observed for NH2-MIL-53 (Al) nanoparticles10b and MIL-101 nanoparticles.10a To our best knowledge, no direct synthesis of an amine-functionalized MIL-101(Cr) framework has been reported.12
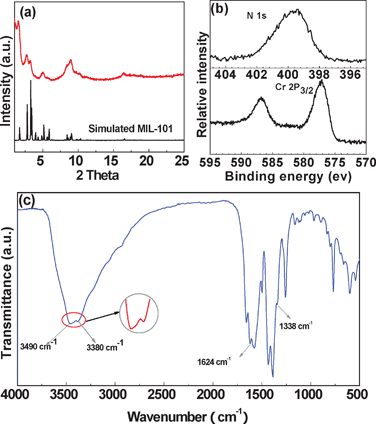 | ||
| Fig. 2 (a) PXRD patterns of as-prepared amine-functionalized MIL-101(Cr) synthesized at 150 °C for 12 h and the simulated pattern for MIL-101(Cr) from ref. 1a. (b) X-ray photoelectron spectra of N 1s and Cr 2p3/2. (c) IR spectra of the resulting products. | ||
To prove the successful functionalization with amine groups, an infrared (IR) analysis of the resulting amine-functionalized MIL-101(Cr) was performed and is presented in Fig. 2c. The double peaks at 3490 cm−1 and 3380 cm−1 were clearly observed, which were ascribed to the asymmetrical and symmetrical stretching vibration adsorption of the amine groups. In the lower frequency region, the 1624 cm−1 and 1338 cm−1 peaks correspond to the N–H bending vibration and the characteristic C–N stretching of aromatic amines, respectively.13 The chemical composition of the amine-functionalized MIL-101(Cr) was further determined to be Cr3O(OH)(H2O)2(C8H5O4N)3·nH2O (n ∼ 3) (Anal. Calcd: C 35.29, N 5.15, H 3.18%; Found: C 34.25, N 5.05, H 3.13%) based on element analysis, which is consistent with the chemical composition of MIL-101(Cr).1a As shown in Fig. 2b, the N 1s and Cr 2p3/2 binding energies of 399.2 eV and 577.1 eV were assigned to PhNH2 and Cr–O in the amine-functionalized MIL-101(Cr), respectively.
We have collected N2 adsorption isotherms on a Micromeritics ASAP 2020 gas adsorption analyzer at 77.3 K to determine the surface area, pore volume and pore structure of the resulting amine-functionalized MIL-101(Cr). As shown in Fig. 3, the completely reversible isotherm exhibited Type-I behavior. We further applied the Brunauer–Emmett–Teller (BET) model to the isotherm for P/P0 between 0.05 and 0.2 (Fig. S3 in the Supporting Information†) and obtained an apparent BET surface area of 1675 m2 g−1 which was comparable to that of reported NH2-MIL-101(Al).8a On the other hand, the Langmuir surface area was calculated to be 2280 m2 g−1. It is clear to see that these values surpass those of most functionalized MOFs.7b Rationally, these values are smaller than that of unfunctionalized MIL-101(Cr), which is caused by the inclusion of amino groups that make some of the framework pores inaccessible to guest molecules. The isotherm clearly shows a very sharp uptake at P/P0 from 10−5 to 10−1, which was a signature feature of the microporous supertetrahedra.14,1a In addition, as shown in the inset of Fig. 3, the isotherm has secondary uptakes at P/P0 ∼ 0.1 and P/P0 ∼ 0.2, indicating the presence of two kinds of nanoporous window in the framework, which is consistent with the MIL-101 structure.
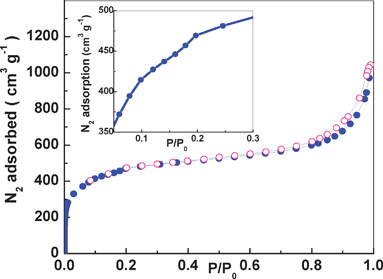 | ||
| Fig. 3 N2 adsorption–desorption isotherms at 77.3 K. The inset figure is the adsorption isotherm in the range of P/P0 = 0.05–0.3. Key: ○ desorption isotherm, ● adsorption isotherm. | ||
To evaluate the stability of as-prepared amine-functionalized MIL-101(Cr), the thermogravimetric and differential thermal analysis (TG-DTA) was performed (Fig. S4 in the Supporting Information†). Two weight-loss steps were observed. The first step (∼6%), corresponding to the loss of free guest water in the pores, occurred in the range 30–200 °C (calculated loss: 7%). The second step (∼71%) was due to the loss of coordinated water molecules, OH groups and the decomposition of the framework between 200 °C and 350 °C (calculated loss: 73%). The thermal stability of the resulting amine-functionalized MIL-101(Cr) was similar to that for MIL-101(Cr) but much higher than that of amine-functionalized MIL-101(Fe) (130 °C).8b It is noteworthy that the resulting amine-functionalized MIL-101(Cr) displayed a good moisture-resistant stability, as the BET surface area almost did not change after exposing the powder to air for 15 days (Fig. S5 in the Supporting Information†). As far as we know, most MOFs are extremely sensitive to moisture, which limits their practical applications.15
Finally, we measured the CO2, CH4 and N2 adsorption isotherms at temperatures of 16 and 25 °C to evaluate the capability of the as-prepared amine-functionalized MIL-101(Cr) as a gas adsorbent. As shown in Fig. 4, the amine-functionalized MIL-101(Cr) displayed higher adsorption capacity for CO2 (up to 15 mmol g−1 at 25 bar) than for N2 and CH4 at both temperatures. The ideal CO2-over-N2 and CO2-over-CH4 selectivities of 8–16 and 3–5 were achieved at 25 °C and tested pressures, respectively. This also implies the inclusion of amine groups in the as-prepared amine-functionalized MIL-101(Cr) because the Lewis basic amine groups can interact strongly with the CO2 molecules.7 In addition, the quadrupole moment16 of CO2 (−14 × 10−40 C m2) is much higher than that of N2 (−4.6 × 10−40 C m2) and CH4 (nonpolar). Thus, both factors lead to the higher uptake of CO2 than of N2 and CH4.17 The amine-functionalized MIL-101(Cr) showed higher adsorption capacity for CH4 over N2. This result is probably ascribed to the larger molecular size for CH4.18 It should be noted that amine-functionalized MIL-101(Cr) displayed excellent adsorption capacity for CO2, up to 25 mmol g−1 at 0.4 °C (Fig. S6 in the Supporting Information†). Moreover, at 1 bar and 0.4 °C, the CO2 capture capacity was 3.3 mmol g−1, and the CO2-over-N2 was 16 (Fig. S7 in the Supporting Information†). For comparison, the original MIL-101 was prepared (Fig. S8 and S9 in the Supporting Information†), and the CO2 adsorption capacity was measured at 0–25 °C. It can be seen that the amine-functionalized MIL-101 displays better CO2 adsorption capacity at low pressure (Fig. S10–S12 in the Supporting Information†), which was due to the mild affinity between CO2 and the amino groups. In addition, the higher CO2 capacity of amine-functionalized MIL-101 was clearly observed at 0.4 °C. To better understand the capture ability of the amine-functionalized MIL-101(Cr) for CO2, we calculated Qst for CO2 using the Clausius–Clapeyron equation (Fig. S13 in the Supporting Information†). Here the amount of CO2 adsorbed was determined using the Langmuir–Freundlich fit for the isotherms as a function of pressure. At the onset of adsorption, Qst was calculated to be ∼50 kJ mol−1, which is similar to that of other amine-functionalized MOFs.7b Thus, the uptake of CO2 at low pressures and high temperatures is ascribed to the strong interaction (i.e., high Qst) between adsorbed CO2 molecules and the Lewis basic amine functionality of the amine-functionalized MIL-101(Cr).
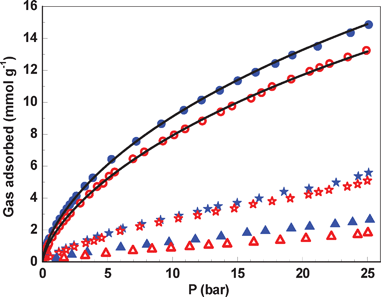 | ||
| Fig. 4 Adsorption isotherms for the amine-functionalized MIL-101(Cr) powder. Key: ● CO2 at 16 °C, ○ CO2 at 25 °C, ★ CH4 at 16 °C, ☆ CH4 at 25 °C, ▲ N2 at 16 °C, Δ N2 at 25 °C. Line: the fitted isotherms for CO2. | ||
In summary, a pure amine-functionalized MIL-101(Cr) with high surface area, good thermal stability and excellent CO2 adsorption capacity has been successfully synthesized by a simple and efficient method. We show that the as-prepared MOF possesses high BET and Langmuir surface areas of up to 1675 and 2280 m2 g−1, respectively. Moreover, the average particle size of the resulting amine-functionalized MIL-101(Cr) was estimated to be around 50 nm, which has been rarely reported in MOF synthesis. In addition, it displayed excellent CO2 capture ability, owing to the synergetic effect of the high surface area and Lewis basic amine groups. We anticipate that amine groups in the as-prepared MOF will offer an opportunity for exploration of further functionality, which may lead to potential applications in the catalysis and separation fields.
Acknowledgements
We acknowledge the financial support from the National Science Foundation of China (Grant No.51072204, 51172249), Technology Innovative Research Program of Ningbo municipality, and the Natural Science Foundation of Zhejiang province (Grant No. Y4100695).References
- (a) G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef; (b) O. K. Farha, A. Özgür Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G.Kanatzidis, S. T.Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944 CrossRef CAS.
- (a) K. M. L. Taylor-Pashow, J. Della Rocca, Z. G. Xie, S. Tran and W. B. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef CAS; (b) P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS; (c) A. Corma, H. Garciía and F. X. Llabreés i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS; (d) H.-L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351 RSC; (e) J. D. Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957 CrossRef; (f) B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115 CrossRef CAS; (g) H.-L. Jiang, Y. Tatsu, Z.-H. Lu and Q. Xu, J. Am. Chem. Soc., 2010, 132, 5586 CrossRef CAS.
- M. Latroche, S. Surblé, C. Serre, C. Mellot-Draznieks, P. L. Llewellyn, J.-H. Lee, J.-S. Chang, S. H. Jhung and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 8227 CrossRef CAS.
- (a) Z. Q. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368–12369 CrossRef CAS; (b) T. Gadzikwa, G. Lu, C. L. Stern, S. R. Wilson, J. T. Hupp and S. T. Nguyen, Chem. Commun., 2008, 5493 RSC.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. Okeeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875 CrossRef CAS.
- T. Gadzikwa, O. K. Farha, K. L. Mulfort, J. T. Hupp and S. T. Nguyen, Chem. Commun., 2009, 3720 RSC.
- (a) S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef CAS; (b) R. Vaidhyanathan, S. S. Iremonger, K. W. Dawson and G. K. H. Shimizu, Chem. Commun., 2009, 5230 RSC.
- (a) J. Gascon, P. Serra-Crespo, E. V. Ramos-Fernandez and F. Kapteijn, Chem. Mater., 2011, 23, 2565 CrossRef; (b) T. Ahnfeldt, D. Gunzelmann, T. Loiseau, D. Hirsemann, J. Senker, G. Ferey and N. Stock, Inorg. Chem., 2009, 48, 3057 CrossRef CAS.
- N. A. Khan, I. J. Kang, H. Y. Seok and S. H. Jhung, Chem. Eng. J., 2011, 166, 1152 CrossRef CAS.
- (a) S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Ferey and J. S. Chang, Adv. Mater., 2007, 19, 121 CrossRef CAS; (b) S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef CAS.
- (a) W. J. Rieter, K. M. L. Taylor, H. Y. An, W. L. Lin and W. B. Lin, J. Am. Chem. Soc., 2006, 128, 9024 CrossRef CAS; (b) H. Xu, F. Liu, Y. J. Cui, B. L. Chen and G. D. Qian, Chem. Commun., 2011, 47, 3153 RSC.
- S. Bernt, V. Guillerm, C. Serre and N. Stock, Chem. Commun., 2011, 47, 2838 RSC.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keefe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef.
- Y. Yoo, V. Varela-Guerrero and H. K. Jeong, Langmuir, 2011, 27, 2652 CrossRef CAS.
- C. Graham, D. A. Imrie and R. E. Raab, Mol. Phys., 1998, 93, 49 CrossRef CAS.
- B. Liu and B. Smit, J. Phys. Chem. C, 2010, 114, 8515 CAS.
- L. Pauling, Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 1967 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20641b |
| This journal is © The Royal Society of Chemistry 2012 |
