Versatile method for bonding hard and soft materials†
Vijaya
Sunkara
,
Dong-Kyu
Park
and
Yoon-Kyoung
Cho
*
School of Nano-Bioscience and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Banyeon-ri 100, Ulsan, Republic of Korea. E-mail: ykcho@unist.ac.kr; Fax: +82-52-217-2509; Tel: +82-52-217-2511
First published on 24th August 2012
Abstract
We report a versatile method for bonding dissimilar materials which is an important issue in the fabrication of micro- and nanofluidic devices. Recently we have demonstrated a simple, surface modification based method for irreversible bonding of thermoplastics to polydimethylsiloxane (PDMS) at room temperature. Here, we present the applicability of this technique for bonding various hard materials including metals and plastics to a soft material like PDMS. An irreversible bonding was formed when the hard material activated by oxygen plasma followed by aminopropyltriethoxy silane (APTES) modification was brought into contact with the plasma treated PDMS and incubated at room temperature. The modified surfaces were characterized by water contact angle measurement, X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM). The analytical data confirms the presence of silane moiety on the treated surface. The tensile strength values of the bonded devices were in the range between 40 kPa∼700 kPa, depending on the type of materials used for bonding. The tested hard materials include gold (Au), platinum (Pt), copper (Cu), iron (Fe), aluminum (Al), polypropylene (PP), high density polyethylene (HDPE) and Teflon and the soft materials include PDMS and butyl rubber.
Introduction
Micro- and nanofluidics offer distinct advantages in various fields for separation and detection of analytes.1–3 For example, the required sample volume and the analysis time are reduced which enables multiplex analysis and high throughput screening with better sensitivity.4 In spite of these advantages, many challenges still remain, some of which are related to device assembly. There is an increasing need for new sealing methods that could facilitate the fabrication of new generation microfluidic devices, which incorporate multi-component structures made of dissimilar materials, not only for biomedical but also for chemical or environmental applications.A considerable amount of literature has been published on device assembly,5–10 which includes adhesive bonding, solvent bonding, thermal bonding etc., each has its advantages and drawbacks. For instance, these bonding techniques could alter the channel dimensions by clogging or change channel wall properties due to the presence of adhesives. Several attempts have been made to avoid channel deformation by using sacrificial materials.11,12 In addition, various surface-modification assisted methods have also been developed for bonding polymer based microfluidic devices.13–16 Furthermore, there are a few reports on irreversible bonding of metal-PDMS, specifically gold using thiol based surface functionalization.17,18 However, thiol based chemistry could be limited to only a few metals that have high affinity to sulphur. Recently, we have demonstrated a surface modification based method for bonding thermoplastic–PDMS hybrid devices.19 The surface modification includes plasma treatment followed by reaction with an organosilane, i.e. 3-aminopropyltriethoxy silane (APTES), at room temperature (RT). An irreversible strong bonding was formed within a few minutes, when the substrates were kept in contact at RT. Furthermore, the bonding showed very high hydrolytic stability even at 37 °C.19
Plasma treatment has been used to alter the surface properties of various materials20–22 and the wettability of the surface was strongly affected by the type of gas employed for the treatment. For example, oxygen plasma23 has been widely used to enhance the wettability and adhesion of PDMS and glass/silicon substrates. Although little is known about the change in surface functional groups after plasma treatment, it is known that the surface energy of the material increases due to the incorporation of oxygen species on the surface. On the other hand, the silane coupling agents have been used as reinforced materials and were also employed to enhance adhesion between polymers and metals in coating industries.24–26
This paper attempts to show that the plasma oxidation in conjunction with surface coating with an aminosilane will increase the adhesion strength of various materials irrespective of their pristine nature or their surface functional groups. We found that this approach is applicable for bonding various hard materials including metals and thermoplastics to a few soft materials like PDMS and butyl rubber. Although the manuscript mainly deals with the hard material-PDMS bonding, the method was also employed for bonding of polycarbonate (PC) and butyl rubber.
Experimental
Materials
All chemicals, reagents and the materials for device fabrication were obtained from commercial sources. PDMS pre-polymer (Sylgard 184) and a curing agent were purchased form Dow Corning (MI, USA). APTES was purchased from Sigma-Aldrich Corp. (MO, USA). The hard materials used were as follows: Au and Pt were purchased from Shin Woo metal, Korea and a 200 nm Au/Pt layer was evaporated onto clean silicon wafer with a 20 nm chromium adhesion layer in an electron beam evaporator; Cu, Fe and Al were from POSCO, Korea and machined at UNIST machine shop; PP, HDPE and butyl rubber were from a local general store and Teflon was from O-Yang Products Co. Ltd., Korea.Surface modification for bonding
All materials were cleaned with isopropyl alcohol except Au and Pt, which were cleaned with piranha, dried and treated with a 60 W oxygen plasma (at 50 sccm oxygen flow rate) for 1 min and placed in an aqueous solution of 1 % v/v APTES for 20 min. The substrates were then washed with DI water and dried under a stream of nitrogen. The materials containing siloxane, e.g. PDMS, were treated with only oxygen plasma (60 W, 50 sccm) for 1 min. The activated substrates were kept in conformal contact at room temperature and the bonding was formed within a few minutes.Effect of surface modification on bonding
Hard materials were cut into 2 cm × 3 cm rectangular pieces, cleaned and dried. Soft materials (PDMS and rubber) were cut into 2 cm × 2 cm pieces. Both of them were treated under various conditions including; (i) oxygen plasma treatment (60 W, 50 sccm, 1 min) and then treatment with 1 % v/v aqueous APTES solution for 20 min; (ii) oxygen plasma treatment (60 W, 50 sccm, 1 min); and (iii) immersed in an aqueous solution of 1 % v/v APTES for 20 min without prior oxygen plasma treatment. All the substrates treated with a reagent were then washed with DI water and blown dried with a stream of nitrogen.Surface characterization
The surfaces of unmodified and modified substrates were assessed by water contact angle measurement using a goniometer (DSA100; KRUSS GmbH, Hamburg, Germany); XPS analysis on a K-alpha analyzer (Thermo Fisher Scientific, UK) and AFM using Multimode V microscope (Veeco, US). The AFM images presented were treated by use of flatten algorithm using the Nanoscope software and average roughness (Ra) was calculated using the software provided by the instrument vendor.Tensile strength measurements
The strength of bonded devices was determined with a tensile strength test. A piece of PDMS (2 × 2 cm, 5 mm thick) was bonded on a piece of hard material (3 × 3 cm Au, Pt, HDPE and PP) or on a 3 × 5 cm piece with holes at four corners (Fe, Cu, Al and Teflon). After bonding, the Au, Pt, HDPE and PP devices were bonded to two aluminum jigs using silicone sealant (LC909N, Henkel). In the case of other devices, the PDMS was bonded to an aluminum jig using silicone sealant and the four corners of the hard materials were clamped to another jig using a bolt and nut (see Fig. S1†). In either cases, the jig was fixed in the tensile strength tester (5848 Micro Tester, Instron, USA) equipped with a 1800 N load sensor. The measurements were recorded at a displacement rate of 1 mm min−1. The force at which the bonded devices failed was recorded and divided by the cross sectional area of the bonded surface to obtain the tensile strength.Results and discussion
Surface modification and bonding strength
Fig. 1 represents the schematic diagram of surface modification and bonding and Fig. 2 shows the images of bonded devices. Various materials including Au, Pt, Cu, Fe, Al, PP, HDPE and Teflon were tested as hard materials and PDMS was used as a soft material for device fabrication. All the hard materials were treated with oxygen plasma followed by an aqueous APTES solution. The surface modified hard materials were kept in contact with the oxygen plasma activated PDMS at RT and the bonding was realized within a few minutes. In the case of PC-butyl rubber bonding, both surfaces were treated with oxygen plasma followed by APTES solution and kept in conformal contact at RT for 1 h.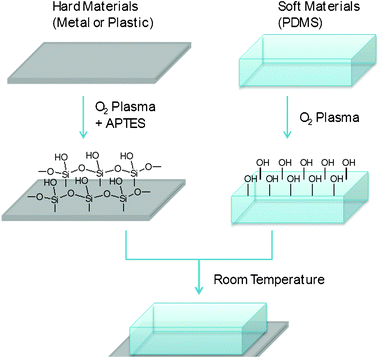 | ||
| Fig. 1 Schematic representation of bonding process. An irreversible bonding was formed between the hard and soft materials within 1 h at room temperature. | ||
 | ||
| Fig. 2 Pictures of various hard material and PDMS bonded devices. | ||
To validate the effect of surface modification on bonding, the materials were treated under different conditions including (i) oxygen plasma activation followed by the treatment with APTES solution; (ii) oxygen plasma activation; and (iii) immersed in APTES solution without prior oxygen plasma activation. Different combinations of the modified substrates were kept in conformal contact at RT for 1 h, bonding was evaluated by manual peeling test and tensile strength measurement. Though the bonding happens within a few minutes after bringing them in contact at RT, the devices were incubated for 1 h to ensure good bonding for all of the different materials tested. The effects of surface treatment on the bonding characteristics are shown in Table 1. The bond strength is classified as strong (S), moderate (M) and weak (W) based on the tensile strength value, which is >200 kPa, 50–200 kPa and <50 kPa for S, M and W respectively. As can be seen from the table, a strong bond was formed in cases where the plasma activated and aminosilane coated hard materials were kept in contact with plasma activated PDMS with or without surface modification. Interestingly, a strong bond was formed between Al and PDMS with only plasma treatment on both. All other surface treatments resulted either in weak bonding or no bonding.
| Hard materials | PDMS | Bonding strengthb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1a | Step 2 | Step 1 | Step 2 | Au | Pt | Cu | Fe | Al | PP | HDPE | Teflon |
| a Plasma: Treated with oxygen plasma (60 W) for 1 min; APTES: immersed in 1 % v/v aq. APTES solution at room temperature (RT) for 20 min; No: no treatment. b The surface treated materials were kept in contact for 1 h at RT and the formation of bonding was evaluated by manual peel tests. Bonding strength: S = Strong (Tensile strength (TS) > 200 kPa); M = Moderate (TS: 50–200 kPa); W = Weak (TS: 20–50 kPa); x = no bonding. The tensile strength over 200 kPa is good enough for conventional microfluidic applications. | |||||||||||
| Plasma | APTES | Plasma | No | S | S | S | S | S | M | M | W |
| APTES | S | S | S | S | W | M | M | W | |||
| No | APTES | Plasma | No | x | x | x | W | x | x | x | x |
| Plasma | No | Plasma | No | x | x | x | x | M | x | x | x |
| APTES | W | W | x | W | W | W | W | x | |||
Quantitative evaluation of the bond strength was done by tensile strength measurement. An image of devices bonded to aluminium jig and the parts released after tensile strength measurement are shown in Fig. S1.† The procedure for device assembly is described in the experimental section and the tensile strength values of various devices are shown in Table 2. The values reported are the average of at least three measurements. All bonded devices exhibit high bond strength, which is a prerequisite for high pressure fluidic applications. The value for gold-PDMS device is in accordance to the previously reported value,17 obtained with other bonding methods and the values for other devices are not available in the literature. To the best of our knowledge, this is the first report that demonstrates a versatile method for bonding different materials at room temperature and reveals their bond strength values.
| Bonded Materiala | Tensile strength (kPa) |
|---|---|
| a For all experiments, PDMS was treated with plasma, just before bonding. b Al–PDMS bonds only with plasma treatment on both surfaces (tensile Strength was 140 ± 21 kPa). c Both PC and rubber were modified with APTES after plasma treatment. The values presented are the average of at least 3 independent measurements. | |
| Au-PDMS | 296 ± 20 |
| Pt-PDMS | 401 ± 19 |
| Cu-PDMS | 312 ± 57 |
| Fe-PDMS | 597 ± 42 |
| Al-PDMSb | 200 ± 28 |
| PP-PDMS | 170 ± 15 |
| HDPE-PDMS | 130 ± 21 |
| Teflon-PDMS | 36 ± 5 |
| PC-Rubberc | 119 ± 7 |
Surface characterization
| Material | Contact Angle (°) | |||
|---|---|---|---|---|
| Pristine | Plasmaa | APTESb | APTES (dry)c | |
| a Treated with oxygen plasma (60 W) for 1 min. b Immersed in 1 % v/v aq. APTES solution at room temperature for 20 min and contact angle was measured immediately after modification. c Same as b, contact angle was measured after 24 h and drying for 10 min under vacuum before measurement. Each value reported was the average of a minimum of five measurements secured at separate positions on any given substrate. | ||||
| Au | 78 ± 2 | 34 ± 1 | 33 ± 2 | 68 ± 1 |
| Pt | 72 ± 1 | 16 ± 1 | 35 ± 1 | 75 ± 1 |
| Cu | 101 ± 2 | 56 ± 2 | 67 ± 1 | 84 ± 1 |
| Fe | 95 ± 2 | 59 ± 1 | 50 ± 1 | 77 ± 1 |
| Al | 103 ± 2 | 35 ± 1 | 48 ± 1 | 80 ± 1 |
| PP | 95 ± 2 | 77 ± 1 | 90 ± 1 | 91 ± 1 |
| HDPE | 101 ± 2 | 61 ± 1 | 55 ± 1 | 78 ± 1 |
| Teflon | 118 ± 3 | 81 ± 1 | 80 ± 1 | 84 ± 1 |
| Rubber | 100 ± 3 | 77 ± 3 | 89 ± 2 | 94 ± 2 |
Surface topographical analysis using AFM
AFM was used to analyze the topographical changes of the unmodified and modified surfaces. Fig. 3 shows the images of pristine (3a and 3d for PP and Au respectively), plasma treated (3b and 3e) and APTES modified (3c and 3f) surfaces. The surface of pristine PP is composed of peaks and valleys yielding large height variation with an average roughness (Ra) of 3.84 nm (see Fig. 3a). Exposure of PP to oxygen plasma led to the formation of several crests and troughs on the surface with heights on the order of a few nanometres (∼10–15 nm), which increased the Ra to 5.27 nm (see Fig. 3b). The increased surface roughness can be ascribed to the attack of oxygen species on the surface during plasma exposure. After aminosilane treatment, the surface exhibits a relatively smooth profile than the plasma treated surface and the Ra is 3.93 nm (see Fig. 3c). This phenomenon indicates that the aminosilane is successfully appended on the PP surface. On the other hand, there was no difference in the topology of Au associated with the surface treatment. The Ra values for pristine, plasma treated and silane modified Au are 2.23, 2.28 and 2.27 nm respectively (Fig 3d–f). | ||
| Fig. 3 Atomic force microscopy (AFM) image (left), 3D image (right top) and surface cross sectional profile (right bottom) of (a) pristine (b) plasma-treated (c) plasma & APTES treated PP and (d) pristine (e) plasma-treated (f) plasma & APTES treated Au (1 μm × 1 μm). | ||
XPS analysis
To assess the change in surface functionality with treatment conditions, the pristine, plasma-treated and APTES-modified PP and Au were analyzed by XPS. General survey spectra and high-resolution spectra for carbon, oxygen, silicon and nitrogen are shown in Fig. 4a and 4b for PP and Au respectively. The atomic compositions were calculated from the C 1s, O 1s, N 1s, Si 2p and Au 4f spectra and are summarized in Table 4. The spectra of pristine PP show O 1s at 532 eV and N 1s at 406 eV, characteristic of NO group, these can be attributed to the adsorbed oxygen or an additive that might be added during the molding process of the thermoplastic. Similarly, presence of C 1s and O 1s in Au can be attributed to the hydrocarbon impurity or adsorbed oxygen on the surface, as their concentration is negligible. In addition, the concentration of N 1s on PP and C 1s on Au are reduced upon exposure to oxygen plasma. Increase in the O 1s concentration on both PP and Au suggests that the oxygen functionalities are incorporated on the surface, which is in agreement with the contact angle measurements. The difference in O 1s to C 1s ratio with treatment condition indicates changes in the surface composition. Initial increase in O1s/C1s ratio after plasma treatment indicates increased oxygen content and a decrease in O1s/C1s ratio after APTES modification suggests increased hydrocarbon content on the surface. Furthermore, the appearance of Si 2p, and N 1s at 400 eV and increase in C 1s concentration after APTES treatment indicates the presence of silane reagent on the surface. | ||
| Fig. 4 XPS spectra for the detection of the atomic concentration of carbon, oxygen, nitrogen and silicon of pristine, plasma treated and APTES-modified PP (a) and Au (b). Peaks for silicon and nitrogen indicate the presence of silane on the surface. | ||
| Material | Treatment condition | Atomic concentration (%) | O1s/C1s | |||
|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Si2p | |||
| PP | Pristine | 89.15 | 9.8 | 1.05 | — | 0.110 |
| Plasma treated | 79.86 | 19.49 | 0.65 | — | 0.244 | |
| APTES modified | 82.26 | 10.86 | 2.16 | 4.71 | 0.132 | |
| Au | Pristine | 12.39 | 3.84 | — | — | 0.310 |
| Plasma treated | 8.43 | 10.71 | — | — | 1.270 | |
| APTES modified | 32.79 | 15.39 | 4.39 | 3.49 | 0.469 | |
The results obtained from the above analyses show that the aminosilane is appended on both thermoplastic and metal surfaces after being exposed to oxygen plasma and APTES treatment. The aminosilane reacts with the hydroxyls of plasma activated PDMS and a strong bond is formed between them through a siloxane (Si–O–Si) bond. The structure and chemistry of aminosilane on the surface of plasma activated thermoplastics and its role in the bonding mechanism was studied extensively and the results will be published elsewhere.
Conclusions
We have demonstrated a versatile method for bonding various hard and soft materials at room temperature. The bonding was facilitated by surface treatment with aminosilane in conjunction with oxygen plasma exposure. All devices show high tensile strength values, indicating an excellent bonding of PDMS to a wide range of substrates including metals such as Au, Pt, Cu, Fe, and Al and thermoplastics such as PP, HDPE, and Teflon. In addition to bonding hard materials with PDMS, the method also showed good bond strength for bonding PC with butyl rubber. In conclusion, the present study suggests that the proposed method is versatile enough to be applicable for bonding various hard and soft materials beyond the simple room temperature bonding method for PDMS and thermoplastics.The method reported here has several advantages besides its versatility such as reproducibility, low cost, compatibility with a variety of materials and ease of handling. Also it does not require high temperature or high pressure and it will not damage the micro features on the substrates. As most of the existing bonding techniques are applicable for a specific type of material, it is advantageous to have a bonding method which could seal all the components simultaneously at low temperature. The method described here has a high potential for concurrent sealing of hybrid devices assembled with active components made of different materials.
Acknowledgements
This work was sponsored by WCU (World Class University) program (R32-2008-000-20054-0) and Basic Science Research Program (2012-0005090) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.References
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS.
- P. S. Dittrich, K. Tachikawa and A. Manz, Anal. Chem., 2006, 78, 3887–3908 CrossRef CAS.
- M. F. Mora, F. Greer, A. M. Stockton, S. Bryant and P. A. Willis, Anal. Chem., 2011, 83, 8636–8641 CrossRef CAS.
- J. Park, V. Sunkara, T.-H. Kim, H. Hwang and Y.-K. Cho, Anal. Chem., 2012, 84, 2133–2140 CrossRef CAS.
- H.-Y. Chen, A. A. McClelland, Z. Chen and J. Lahann, Anal. Chem., 2008, 80, 4119–4124 CrossRef CAS.
- R. T. Kelly and A. T. Woolley, Anal. Chem., 2003, 75, 1941–1945 CrossRef CAS.
- S. Yi, K. Yien Chian and N. Nam-Trung, J. Micromech. Microeng., 2006, 16, 1681–1688 CrossRef.
- P. Yu-Jen and Y. Ruey-Jen, J. Micromech. Microeng., 2006, 16, 2666–2672 CrossRef.
- M. T. Koesdjojo, C. R. Koch and V. T. Remcho, Anal. Chem., 2009, 81, 1652–1659 CrossRef CAS.
- P. Abgrall, L.-N. Low and N.-T. Nguyen, Lab Chip, 2007, 7, 520–522 RSC.
- M. T. Koesdjojo, Y. H. Tennico and V. T. Remcho, Anal. Chem., 2008, 80, 2311–2318 CrossRef CAS.
- R. T. Kelly, T. Pan and A. T. Woolley, Anal. Chem., 2005, 77, 3536–3541 CrossRef CAS.
- L. Brown, T. Koerner, J. H. Horton and R. D. Oleschuk, Lab Chip, 2006, 6, 66–73 RSC.
- C.-W. Tsao and D. DeVoe, Microfluid. Nanofluid., 2009, 6, 1–16 CrossRef CAS.
- Y. H. Tennico, M. T. Koesdjojo, S. Kondo, D. T. Mandrell and V. T. Remcho, Sensor. Actuat. B-Chem., 2010, 143, 799–804 CrossRef.
- M. E. Vlachopoulou, A. Tserepi, P. Pavli, P. Argitis, M. Sanopoulou and K. Misiakos, J. Micromech. Microeng., 2009, 19, 015007 CrossRef.
- E. Ouellet, C. W. T. Yang, T. Lin, L. L. Yang and E. T. Lagally, Langmuir, 2010, 26, 11609–11614 CrossRef CAS.
- K. J. Lee, K. A. Fosser and R. G. Nuzzo, Adv. Funct. Mater., 2005, 15, 557–566 CrossRef CAS.
- V. Sunkara, D.-K. Park, H. Hwang, R. Chantiwas, S. A. Soper and Y.-K. Cho, Lab Chip, 2011, 11, 962–965 RSC.
- W.-K. Kim and J.-L. Lee, Appl. Phys. Lett., 2006, 88, 262102–262103 CrossRef.
- E. M. Liston, L. Martinu and M. R. Wertheimer, J. Adhes. Sci. Technol., 1993, 7, 1091–1127 CrossRef CAS.
- J. Chai, F. Lu, B. Li and D. Y. Kwok, Langmuir, 2004, 20, 10919–10927 CrossRef CAS.
- J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H. Wu, O. J. A. Schueller and G. M. Whitesides, Electrophoresis, 2000, 21, 27–40 CrossRef CAS.
- M. L. Abel, J. F. Watts and R. P. Digby, Int. J. Adhes. Adhes., 1998, 18, 179–192 CrossRef CAS.
- E. P. Plueddemann, Silane coupling agents, Plenum Press, 1991 Search PubMed.
- K. L. Mittal, Silanes and other coupling agents, VSP, 2000 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Figure S1 and S2. See DOI: 10.1039/c2ra20880f/ |
| This journal is © The Royal Society of Chemistry 2012 |
