One-pot depolymerization of cellulose into glucose and levulinic acid by heteropolyacid ionic liquid catalysis†
Zhong
Sun
a,
Mingxing
Cheng
a,
Huacheng
Li
a,
Tian
Shi
a,
Mengjia
Yuan
a,
Xiaohong
Wang
*a and
Zijiang
Jiang
*b
aKey Lab of Polyoxometalate Science of Ministry of Education, Faculty of Chemistry, Northeast Normal University, Changchun, 130024, P. R. China. E-mail: wangxh665@nenu.edu.cn; Fax: 0086-431-85099759; Tel: 0086-431-88930042
bChangchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, P. R. China. E-mail: jzj2002@sohu.com; Tel: 0086-431-85262452
First published on 31st July 2012
Abstract
A series of heteropolyacid (HPA) ionic liquids [C4H6N2(CH2)3SO3H]3−nHnPW12O40 ([MIMPSH]nH3−nPW12O40, n = 1, 2 3, abbreviated as [MIMPSH]nH3−nPW) was used to catalyze one-pot depolymerization of cellulose into glucose. Their performances were much better than those of the previously reported HPAs, such as H3PW12O40, Cs2.5H0.5PW12O40. Besides cellulose, the HPA ionic liquids were able to catalyze the conversion of sucrose and starch into glucose. In addition, one-pot synthesis of levulinic acid (LA) directly from cellulose was realized using these HPA ionic liquid catalysts in a water–methyl isobutyl ketone (MIBK) biphasic system. The separation of the products and catalysts was easy, and the retrieved [MIMPSH]nH3−nPW could be repeatedly used without appreciable loss of performance.
1. Introduction
Cellulose is a kind of polysaccharide and the most plentiful source in nature, which holds impressive potential as an alternative to fossil-based fuels.1,2 One-pot conversion of cellulose into small organic molecules under acidic, basic, oxidative, reductive and hydrothermal conditions has been reported,3 in which monosaccharides, 5-hydroxymethylfurfural (HMF), lactic acid, levulinic acid (LA) and polyols such as sorbitol have been obtained. However, cellulose not only provides a renewable carbon source, but also offers challenges to researchers due to the structural recalcitrance.4 Considerable efforts have been made to convert cellulose into biofuels and feedstock chemicals. Diluted acids are suitable catalysts for the hydrolysis of cellulose with higher glucose yields.5 However, the generation of acidic waste and equipment corrosion are the significant drawbacks hindering the application. Thus, the alternative ways to achieve cellulose depolymerization are developed, such as hydrolysis in ionic liquids or supercritical media. Recently, the use of heterogeneous catalysis was successfully developed to promote cellulose depolymerization.Onda et al. found that sulfonated activated carbon exhibited higher activity for cellulose hydrolysis into glucose with a yield of 41% at 423 K and 3 h.6,7 Zhang et al. investigated the sulfonated carbon with a mesoporous structure, which exhibited an obviously high glucose yield (75%) at 423 K and 24 h, which is the highest recorded yield on a solid acid.8 The magnetic solid acid catalyst Fe3O4-SBA-SO3H for conversion of amorphous cellulose was achieved by Lai et al., giving a 50% yield of glucose at 423 K and 3 h.4 Recently, Smet's group reported that the sulfonated polymer acid-catalyst led to an LA yield of approximately 30 mol% after reaction for 3 h at 443 K or 5 h at 438 K.9 Apart from sulfonic material, the layered transition metal oxide HNbMoO6 exhibited a remarkable catalytic performance for hydrolysis of saccharides including cellobiose and cellulose,10 although the yield of glucose from cellulose and starch was low at 403 K and 12 h. Heteropolyacids (HPAs) or salts are known to exhibit activity for the hydrolysis of polysaccharides including disaccharides, starch and cellulose.12–15 Mizuno16 reported that the highest yield of glucose had been achieved using concentrated H5BW12O40 (0.7 M) as the catalyst, at 333 K and 48 h, such as 82% total reduced sugar yield and 77% glucose yield based on cellulose. In the same report, one-pot synthesis of LA from cellulose was realized to obtain a 60% yield using the same catalyst H5BW12O40. Just at the same time, Chambon's group reported that using heterogeneous Brønsted and Lewis acids could promote the depolymerization of cellulose and favor the production of lactic acid.17 Both H3PW12O40 and CsHPAs have been very successfully used in the hydrolytic hydrogenation of cellulose to polyols.18
Although some solid catalysts have been reported to catalyze cellulose saccharification (Table S1, ESI†), the systems require higher catalyst/substrate mass ratios,19 in many cases more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, high temperatures and long times, resulting in a lowered selectivity to glucose. The most important reason for the above problems is the insolubility of cellulose in any solvent, leading to difficulties in solid to solid mass transport. Therefore, heterogeneous catalysis cannot be easily applied to solid–solid catalysis systems due to the difficulty of mass transport. Fukuoka pointed out that “this may by solved by having freely available protons in the vicinity of the catalyst and the substrate”.2 So there is still a need for improved processes for highest total yield of saccharides, selectivity to glucose, lower cost and higher energy efficiency under milder conditions using solid acid catalysts.
1, high temperatures and long times, resulting in a lowered selectivity to glucose. The most important reason for the above problems is the insolubility of cellulose in any solvent, leading to difficulties in solid to solid mass transport. Therefore, heterogeneous catalysis cannot be easily applied to solid–solid catalysis systems due to the difficulty of mass transport. Fukuoka pointed out that “this may by solved by having freely available protons in the vicinity of the catalyst and the substrate”.2 So there is still a need for improved processes for highest total yield of saccharides, selectivity to glucose, lower cost and higher energy efficiency under milder conditions using solid acid catalysts.
Based on the above knowledge, we prepared a kind of HPA ionic liquid (HPA IL) [MIMPSH]nH3−nPW (n = 1, 2) to achieve the conversion of cellulose into glucose under mild conditions. [MIMPSH]3PW is a kind of HPA IL, which was first synthesized by Prof. Wang21 and used as a heterogeneous catalyst in an esterification reaction. And more recently, Prof. Huang's group tried to achieve the dehydration of fructose into HMF using the recyclable catalyst [MIMPSH]3PW, which obtained a 99.1% yield of HMF at 120 °C after 2 h.32 Using an analogous method, we prepared a series of [MIMPSH]nH3−nPW (n = 1, 2) containing different numbers of protons. This kind of catalyst contains the Brønsted acidity from the HPA part, which favors the dehydration of cellulose. In addition, the cation part acts like an ionic liquid, which could promote the dissolution of the cellulose molecules, hence increasing the reaction rate.33 We have previously reported the conversion of cellulose catalyzed by micellar HPA catalysts such as [C16H33N(CH3)3]H2PW12O40.26 Compared to our previous report, IL type HPA catalysts are soluble in water to form aqueous solutions, providing more availability for the transformation of cellulose into a catalyst. [MIMPSH]nH3−nPW exhibited higher activity than micellar HPA catalysts and could be recyclable.
2. Experimental
2.1 Materials and characterization techniques
Microcrystalline cellulose (white, average particle size 50 μm) was obtained from J&K Chemical Ltd (Beijing, China). The X-ray diffraction analysis was carried out on a Japan Rigaku Dmax 2000 X-ray diffractometer with Cu-Kα radiation (λ = 0.154178 nm) to analyze the structure of cellulose, and the degree of cellulose crystallinity was about 0.6. The degree of polymerization was 300 measured by viscosity. H3PW12O40·23H2O was prepared according to the literature method.22 All other reagents were of AR grade and were used without further purification. 3,5-Dinitrosalicylic acid (DNS) reagent was prepared according to ref. 23.2.2 Preparation of [MIMPSH]nH3−nPW12O40 (Scheme 1)
Preparation of [C4H6N2(CH2)3SO3H]3PW12O40 followed Wang's report.21 Under a nitrogen atmosphere, methylimidazole (0.11 mol) and 1,3-propane sulfone (0.10 mol) were dissolved in toluene (20 mL) and stirred for 24 h at 50 °C. A white precipitate MIMPSH formed, which was filtered and washed with diethyl ether three times, then dried in a vacuum. MIMPSH (0.06 mol) was added to an aqueous solution of H3PW12O40 (0.02 mol), and then the mixture was stirred at room temperature for 24 h. The water was then removed in a vacuum to give the solid product. The white solid was washed with water and ethanol to remove unreacted substrates and dried at 100 °C for about 3 h. The catalyst was characterized by FTIR, 1H and 31P NMR spectroscopy. The resulting [MIMPSH]3PW12O40 was obtained with yield 46%. Mp: 120–122 °C. IR (1% KBr pellet, 4000–400 cm−1, a Nicolet Magna 560 IR spectrometer): 1079, 979, 898 and 801 cm−1. Anal. calcd for [C4H6N2(CH2)3SO3H]3PW12O40: W, 63.16; P, 0.89; S, 2.75; C, 7.22; H, 1.12; N, 2.41%. Found: W, 63.38; P, 0.76; S, 2.59; C, 7.43; H, 1.23; N, 2.33% (elemental analysis was carried out using a Leeman Plasma Spec (I) ICP-ES and a PE 2400 CHN elemental analyzer). 1H NMR (A INOVA 500 NMR in CDCl3): δ 2.221 (m, 2H), 2.833 (t, 2H), 3.946 (s, 3H), 4.208 (t, 2H), 4.671 (s, 1H), 7.433 (d, 1H), 7.477 (d, 1H), 8.602 (s, 2H). 31P MAS NMR: −16.51 ppm.![The synthesis of [MIMPSH]H2PW12O40.](/image/article/2012/RA/c2ra01328b/c2ra01328b-s1.gif) | ||
| Scheme 1 The synthesis of [MIMPSH]H2PW12O40. | ||
The preparation of [C4H6N2(CH2)3SO3H]2HPW12O40 and [C4H6N2(CH2)3SO3H]H2PW12O40 is similar to the preparation of [C4H6N2(CH2)3SO3H]3PW12O40, except that the molar ratios of MIMPSH to H3PW12O40 are 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The resulting [MIMPSH]2HPW12O40 was obtained with yield 48%. Mp: 123–126 °C. IR: 1080, 983, 887, and 796 cm−1. Anal. calcd for [C4H6N2(CH2)3SO3H]2HPW12O40: W, 67.08; P, 0.94; S, 1.95; C, 5.11; H, 0.83; N, 1.70%. Found: W, 67.17; P, 1.03; S, 2.02; C, 5.07; H, 0.89; N,1.79%. 1H NMR: δ 2.203 (m, 2H), 2.811(t, 2H), 3.932 (s, 3H), 4.185 (t, 2H), 4.606 (s, 1H), 7.432 (d, 1H), 7.486 (d, 1H), 8.654 (s, 2H). 31P MAS NMR: −16.42 ppm. The resulting [MIMPSH]H2PW12O40 was obtained with yield 46%. Mp: 127–129 °C. IR (1% KBr pellet, 4000–400 cm−1): 1080, 982, 890 and 799 cm−1. Anal. calcd for [C4H6N2(CH2)3SO3H]H2PW12O40: W, 71.53; P, 1.00; S, 1.04; C, 2.73; H, 0.49; N, 0.91%. Found: W, 71.48; P, 1.03; S, 0.95; C, 2.47; H, 0.58; N, 0.83%. 1 H NMR: δ 2.211 (m, 2H), 2.842 (t, 2H), 3.935 (s, 3H), 4.252 (t, 2H), 4.666 (s, 1H), 7.426 (d, 1H), 7.470 (d, 1H), 8.613 (s, 2H). 31P MAS NMR: −16.31 ppm. 13C NMR: δ 14.685, 23.418, 27.083, 30.503, 32.647, 54.258, 68.367 ppm.
2, respectively. The resulting [MIMPSH]2HPW12O40 was obtained with yield 48%. Mp: 123–126 °C. IR: 1080, 983, 887, and 796 cm−1. Anal. calcd for [C4H6N2(CH2)3SO3H]2HPW12O40: W, 67.08; P, 0.94; S, 1.95; C, 5.11; H, 0.83; N, 1.70%. Found: W, 67.17; P, 1.03; S, 2.02; C, 5.07; H, 0.89; N,1.79%. 1H NMR: δ 2.203 (m, 2H), 2.811(t, 2H), 3.932 (s, 3H), 4.185 (t, 2H), 4.606 (s, 1H), 7.432 (d, 1H), 7.486 (d, 1H), 8.654 (s, 2H). 31P MAS NMR: −16.42 ppm. The resulting [MIMPSH]H2PW12O40 was obtained with yield 46%. Mp: 127–129 °C. IR (1% KBr pellet, 4000–400 cm−1): 1080, 982, 890 and 799 cm−1. Anal. calcd for [C4H6N2(CH2)3SO3H]H2PW12O40: W, 71.53; P, 1.00; S, 1.04; C, 2.73; H, 0.49; N, 0.91%. Found: W, 71.48; P, 1.03; S, 0.95; C, 2.47; H, 0.58; N, 0.83%. 1 H NMR: δ 2.211 (m, 2H), 2.842 (t, 2H), 3.935 (s, 3H), 4.252 (t, 2H), 4.666 (s, 1H), 7.426 (d, 1H), 7.470 (d, 1H), 8.613 (s, 2H). 31P MAS NMR: −16.31 ppm. 13C NMR: δ 14.685, 23.418, 27.083, 30.503, 32.647, 54.258, 68.367 ppm.
2.3 Catalytic procedure
For hydrolysis of cellulose, a mixture of cellulose (0.1 g) and catalyst (0.07 mmol) was added into water (0.5 mL) and methyl isobutyl ketone (MIBK, 5 mL), then the mixture was heated at 140 °C in a steel autoclave lined with Teflon under air for 5 h with stirring (300 rpm). The reaction was stopped by rapidly cooling the reactor in an ice bath at 0 °C. The reaction mixture was separated into three layers: the upper layer is the organic solvent containing small organic molecules such as HMF or LA; the middle layer is water containing the catalyst and TRS, the lower layer is unreacted cellulose. The sugars were precipitated from the aqueous phase by adding excess ethanol to the aqueous phase of the reaction mixtures, followed by separation of the carbohydrate (solid) by filtration. Then the solid catalyst was recovered by evaporation of the solvents. Cellulose conversions (wt%) were determined by the change in cellulose weight before and after the reaction. Then the unreacted cellulose was identified by IR spectroscopy.The concentration of glucose (g mL−1) was measured in the aqueous phase by high-performance liquid chromatography (HPLC), which was conducted on a system equipped with a refractive index detector (Shimadzu LC-10A, HPX-87H column). The concentrations of 5-hydroxymethylfurfural and levulinic acid in the MIBK phase were determined by gas chromatography (Agilent 6890) equipped with an Agilent 19091J-416 capillary column and flame ionization detector. Qualitative analysis was performed by gas chromatography-mass spectrometry (GC-MS, Agilent 5970) with scan parameters: low mass 20.0, high mass 700.0, and threshold 150.
2.4 Total reducing sugars (TRS) analysis
A mixture that contained 0.8 mL of DNS reagent and 0.4 mL of the reaction sample was heated for 2 min in a boiling water bath, then cooled to room temperature by flowing water and mixed with deionized water to 10 mL. The color intensity of the mixture was measured in a UV757CRT Model spectrophotometer at 540 nm. The concentration (g mL−1) of total reducing sugars was calculated based on a standard curve obtained with glucose.2.5 Hammett acidity (H0) analysis
A sample of the catalyst (100 mg) and Hammett indicator (p-nitroaniline, pK(I)aq = 0.99, 50 mg) were dissolved in 20 mL secondary distilled water. The mixture was stirred for 12 h in the sealed reactor at room temperature, and then the solid was separated by filtration. The color intensity of the mixture was measured in a Cary 500 UV/Vis/NIR spectrophotometer at 380 nm.3. Results and discussion
3.1. Characterization of the catalysts
From the elemental analysis data, the results were satisfactory with the calculated values.The IR spectra of [MIMPSH]nH3−nPW12O40 (Fig. S1, ESI†) showed four characteristic peaks at about 1080, 980, 896 and 806 cm−1, reflecting the four different vibrations in the Keggin-type structure PW12O403−, which could be attributed to the asymmetry vibrations P–Oa (internal oxygen connecting P and W), W–Od (terminal oxygen bonding to W atom), W–Ob (edge-sharing oxygen connecting W) and W–Oc (corner-sharing oxygen connecting W3O13 units). These results show that the as-prepared materials contain PW12O403−.
The 31P MAS NMR spectra of [MIMPSH]nH3−nPW12O40 show one signal peak at δ = −16.51, −16.42, and −16.31 ppm, respectively, corresponding to [MIMPSH]3PW, [MIMPSH]2HPW, and [MIMPSH]H2PW, whereas the H3PW12O40·6H2O gives a peak at −15.6 ppm. The shifts of 31P MAS NMR were attributed to the introduction of organic cations into H3PW12O40, which could confirm the formation of [MIMPSH]nH3−nPW12O40 and not the physical mixture of [MIMPSH] and PW12O403−. (The 31P MAS NMR spectrum of the physical mixture of [MIMPSH] and H3PW12O40 is at −15.3 ppm.) The different peaks of 31P NMR among the three catalysts were attributed to the different number of organic groups. Compared to the 1H NMR of MIMPSH,20 the 1H NMR of [MIMPSH]nH3−nPW12O40 also changed, also showing the formation of [MIMPSH]nH3−nPW12O40. The 13C NMR of [MIMPSH]nH3−nPW12O40 could determine the existence of organic parts in [MIMPSH]nH3−nPW12O40. δ = 14.685, 27.083, 30.503 could determine the existence of organic parts in –CH2–CH2–CH2–, and δ = 23.418, 32.647, 54.258 could determine the existence of organic parts in C4H6N2–.
3.2. Hydrolysis of cellulose with various catalysts
The activity of HPA based IL catalysts was studied through cellulose hydrolysis (Table 1) and the main product is glucose. It can be seen that C4H6NN+(CH2)3SO3− is a kind of IL without any acid sites, which exhibits no activity for cellulose hydrolysis under such reaction conditions. H3PW12O40 is a homogeneous acid catalyst, which is favorable for the conversion of cellulose. However, the yield of glucose is low, owing to the high acid strength of the catalyst, leading to further degradation of glucose into small organic molecules, such as HMF and LA. Cs2.5H0.5PW12O40 as a heterogeneous acid catalyst shows low catalytic activity, and the yields of TRS and glucose reach 10.0% and 8.1%, respectively. Using the same concentrations of C4H6NN+(CH2)3SO3− and H3PW12O40 (entry 3), the conversion of cellulose is almost the same as that by H3PW12O40. This indicates that the catalytic activity mainly comes from HPAs. H3PW12O40 dissolved in the mixture of water and C4H6NN+(CH2)3SO3−, which leads to a higher conversion but a lower yield of glucose in the water system.| Entry | Catalysts | Conversion/wt% | TRS/wt% | Glucose/wt% | TOFd/g mmol−1 |
|---|---|---|---|---|---|
| a Reaction conditions: 0.1 g cellulose with 0.07 mmol catalyst, 0.5 mL water and 5 mL MIBK at 140 °C in 5 h. b Reaction conditions: 0.1 g cellulose with 0.07 mmol H3PW12O40 and 0.07 mmol IL as the catalyst in 0.5 mL water and 5 mL MIBK at 140 °C in 5 h. c There is no MIBK, only with 5.5 mL water. d TOF = the yield of glucose/the concentration of protons (considering the proton totally dissociated. It is 3 in entries 2–7.) | |||||
| 1 | MIMPSH | 0 | 0 | 0 | 0 |
| 2 | H3PW12O40 | 54.1 | 31.6 | 27.0 | 0.12 |
| 3 | H3PW12O40 + ILb | 57.3 | 33.8 | 27.1 | 0.12 |
| 4 | [MIMPSH]H2PW | 55.1 ± 1.7 | 40.2 ± 1.7 | 36.0 ± 2.6 | 0.17 |
| 5 | [MIMPSH]H2PWc | 13.1 ± 1.6 | 9.6 ± 2.0 | 8.4 ± 2.3 | 0.14 |
| 6 | [MIMPSH]2HPW | 42.7 ± 1.3 | 35.9 ± 1.9 | 27.3 ± 1.8 | 0.13 |
| 7 | [MIMPSH]3PW | 28.4 ± 1.4 | 25.2 ± 1.5 | 23.2 ± 2.5 | 0.11 |
| 8 | Cs2.5H0.5PW12O40 | 12.3 | 10.0 | 8.1 | |
[MIMPSH]H2PW exhibits lower conversion but higher glucose yield compared to H3PW12O40. The conversion of cellulose and the yield of glucose are 55.1% and 36.0%, respectively, for reactions of 5 h. The effects of [MIMPSH]H2PW in the hydrolysis reaction can be considered as two parts. One is the solubility of the IL HPA catalyst in water to form homogeneous solution, which is available for conversion of cellulose. The other is the strong Brønsted acidity from H3PW12O40.
The Brønsted acidity of different catalysts can be measured by using a UV-indicator.24 The lower H0 is, the stronger the acidity of the catalyst. Fig. 1 gives the H0 values of different catalysts. It can be seen that the H0 value would increase with increasing ratio of MIMPSH to H3PW12O40. The acid strength is H3PW12O40 ∼ [MIMPSH]H2PW > [MIMPSH]2HPW > [MIMPSH]3PW.
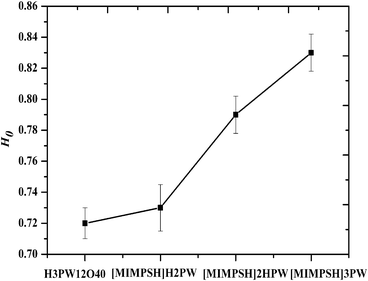 | ||
| Fig. 1 The H0 values (mmol g−1) of different catalysts. | ||
The relationship between Brønsted acidity and catalytic activity of the IL HPA system is that the Brønsted acidity influences the conversion of cellulose and the yield of glucose as well. These catalytic results follow the order [MIMPSH]H2PW > [MIMPSH]2HPW > [MIMPSH]3PW (Fig. 2). It can be seen that the yields of glucose are influenced by the Brønsted acidities of the different IL HPA catalysts. The conversion of cellulose reaches almost 100% catalyzed by [MIMPSH]H2PW for about 12 h, and this catalyst also gives the best yield of glucose among the three catalysts (Fig. 2), with a shorter time (5 h). In addition, the yield of glucose increases in the beginning, then decreases with a further increase in reaction time. The total yields of organic molecules (including glucose, HMF and LA) differ among the three catalysts, while [MIMPSH]H2PW gives the highest total yield (83.8%) among the three catalysts (Fig. 3). The high Brønsted acidities of [MIMPSH]H2PW and [MIMPSH]2HPW could enhance the yield of LA but decrease the yield of glucose by increasing the reaction time. The yield of LA was higher for [MIMPSH]H2PW than for [MIMPSH]2HPW. [MIMPSH]3PW, with the lowest Brønsted acidity, gives low yields of LA and HMF as well. During the hydrolysis of cellulose by [MIMPSH]H2PW or [MIMPSH]2HPW, the obtained HMF further transforms into LA. It is known that HMF and LA are both potentially versatile building blocks for the synthesis of different chemicals. HMF is produced by acid-catalyzed dehydration of carbohydrates and LA is a product of the subsequent rehydration of HMF.3 Our study demonstrates that an increase in acidic strength of the catalysts could accelerate the conversion of HMF to LA, and a considerably high amount of LA (63.1%) is obtained by [MIMPSH]H2PW.
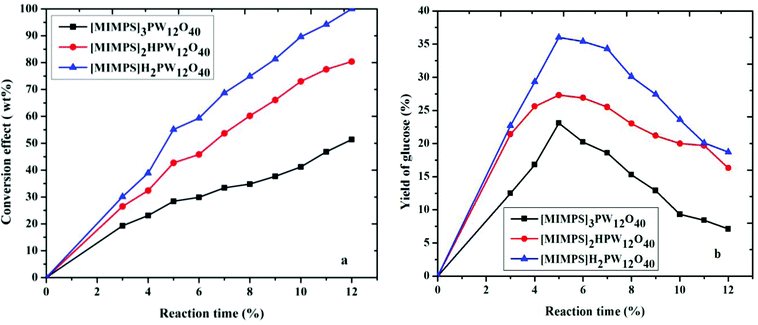 | ||
| Fig. 2 Hydrolysis of cellulose by different catalysts. Reaction conditions: 0.1 g cellulose with 0.07 mmol catalyst, 0.5 mL water and 5 mL MIBK at 140 °C. | ||
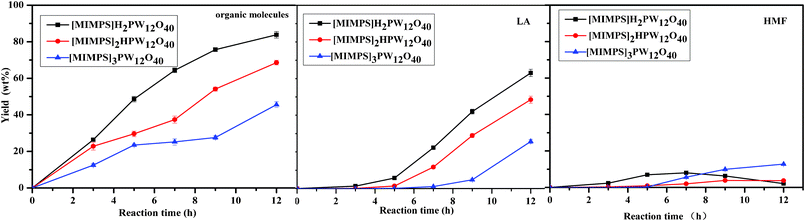 | ||
| Fig. 3 The different products obtained by the different IL HPA catalysts with different reaction times. Reaction conditions: 0.1 g cellulose with 0.07 mmol catalyst, 0.5 mL water and 5 mL MIBK at 140 °C. Organic molecules include glucose, HMF, and levulinic acid. | ||
The amount of water could influence the conversion of cellulose, because altering the water content leads to a change in catalyst concentration.25,26 The reaction was performed in 5.5 mL water (Table 1, entry 5). The conversion of cellulose is only about 13.1%, and the yields of glucose and TRS are 8.4% and 9.6%, respectively. MIBK had been chosen for the hydrolysis of cellulose, where MIBK is an organic solvent that could act as extractive phase available for the organic molecules to enhance the conversion of cellulose.27 The organic molecules tested in our experiment were glucose, HMF, and LA. In addition, the structure of the cellulose molecule did not change during the dehydration reaction (Fig. S3, ESI†).
3.2. Influence of reaction conditions on cellulose hydrolysis
The influence of catalyst amounts on the hydrolysis of cellulose was investigated by varying the amount of [MIMPSH]H2PW ranging from 0.05 mmol to 0.09 mmol (Fig. 4). As expected, the more catalyst there is, the higher the conversion of cellulose. The conversion of cellulose is enhanced from 46.2% to 65.8% in 5 h. The yields of glucose and TRS could respectively reach to maxima of 31.1% and 40.2% with 0.07 mmol catalyst. Increasing the amount of catalyst further, i.e. 0.08 mmol, the yields of glucose, TRS, and HMF begin to decrease. Meanwhile, the yield of levulinic acid increases. This result also demonstrates that a higher concentration of the catalyst leads to glucose being converted into HMF, and then further into LA. In order to obtain a high glucose yield, a smaller amount of catalyst (0.07 mmol) could be used.![The effect of [MIMPSH]H2PW dosages on cellulose hydrolysis. Reaction conditions: 0.1 g cellulose, 0.5 mL water and 5 mL MIBK at 140 °C in 5 h.](/image/article/2012/RA/c2ra01328b/c2ra01328b-f4.gif) | ||
| Fig. 4 The effect of [MIMPSH]H2PW dosages on cellulose hydrolysis. Reaction conditions: 0.1 g cellulose, 0.5 mL water and 5 mL MIBK at 140 °C in 5 h. | ||
The reaction time influences the yields of glucose, HMF and LA (Fig. 5). It can be seen that the yield trends of glucose and HMF increase first, then decrease. The yields of glucose and HMF reach their highest values at 5 h and 7 h, respectively. On the contrary, the yields of LA increase as the reaction time is prolonged. The LA yield reaches 63.1% at 12 h. This result demonstrates that the conversion of glucose to LA could be obtained by acid-catalysis with a long reaction time.
![The effect of reaction time on cellulose hydrolysis. Reaction conditions: 0.1 g cellulose with 0.07 mmol [MIMPSH]H2PW, 0.5 mL water and 5 mL MIBK at 140 °C.](/image/article/2012/RA/c2ra01328b/c2ra01328b-f5.gif) | ||
| Fig. 5 The effect of reaction time on cellulose hydrolysis. Reaction conditions: 0.1 g cellulose with 0.07 mmol [MIMPSH]H2PW, 0.5 mL water and 5 mL MIBK at 140 °C. | ||
We also studied the effect of the reaction temperature on the conversion of cellulose. It is found that reaction temperature has a great effect on both conversion and yield (Fig. 6). The times for maximum yields of TRS and glucose are different, based on the different reaction temperatures; that is, the higher the temperature is, the shorter the reaction time needs to be. At the higher temperature of 140 °C, the yields of TRS and glucose reach maximum values of 40.6% and 36.0% in 5 h. The TRS and glucose yields decrease to 24.5% and 20.1% at 130 °C in the same time. For a reaction temperature of 120 °C, the reaction time was much longer as the yields of TRS and glucose reach maximum values. It could also be seen that the maximum LA yield of 22.3% is obtained at 140 °C with a reaction time of 7 h. At 120 °C, LA yield is only 1.4% for 7 h. This result shows that production of LA needed a higher temperature and a longer reaction time.
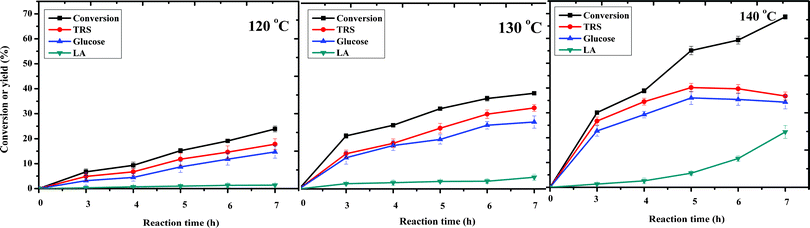 | ||
| Fig. 6 The effect of temperature on cellulose hydrolysis. Reaction conditions: 0.1 g cellulose with 0.07 mmol catalyst, 0.5 mL water and 5 mL MIBK at different temperatures. | ||
3.3 Recycling of the catalyst
Reusability of the catalyst is very important in practical applications. IL HPA catalysts have high solubility in alcoholic solvents, such as ethanol. In contrast, saccharides (mainly glucose) are almost insoluble in ethanol.15 Thus, here we discuss the recycling of both the catalyst and the solvent in cellulose hydrolysis reactions. Based on the different solubilities of the HPA catalyst and product in ethanol, we can separate them completely. After the hydrolysis reaction finished, the aqueous solution (containing TRS and the catalyst) was extracted by ethanol to separate the HPAs twice, and then the ethanol was removed by evaporation to give the solid catalyst (98% recovery). The IR spectra of H3PW12O40 and [MIMPSH]H2PW (fresh and recycled) are shown in Fig. S1, (ESI†). The IR spectrum of [MIMPSH]H2PW (recycled) is in good agreement with that of its parent H3PW12O40 and [MIMPSH]H2PW (fresh), showing that it retains Keggin structure after recovery. No solid humins were found in the IR spectrum, indicating no humin formed during the reaction. The color of the cellulose and refreshed catalyst is pale white. The total amounts of W and C in the water media during six recycling reaction were only 0.8% and 1.0% corresponding to the W and C contents of the fresh catalyst.The retrieved [MIMPSH]H2PW could be used repeatedly without an appreciable loss of its high activity (Fig. 7) after six times.
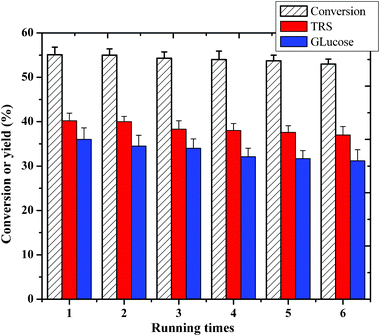 | ||
| Fig. 7 Recycling of the IL HPA catalyst for hydrolysis of cellulose. Reaction conditions: 0.1 g cellulose with 0.07 mmol catalyst, 0.5 mL water and 5 mL MIBK at 140 °C for 5 h. | ||
3.4 Conversion of sucrose and starch to glucose
Besides cellulose, the present system was also applicable in the conversion of other polysaccharides, such as sucrose and starch. The conversion of feedstocks and the yield of glucose are given in Fig. 8. From the results, it can be seen that saccharification efficiently proceeds by [MIMPSH]H2PW, giving glucose in high yields (>90%). Compared to our previous reports,25,26 the IL HPA catalyst provided a higher efficiency in the transformation of polysaccharides into water-soluble products.![Transformation of polysaccharides (1 g) by [MIMPS]H2PW12O40 (0.077 mmol, 0.5 mL water, 5.0 mL MIBK). The conversion and yield are given in the figure.](/image/article/2012/RA/c2ra01328b/c2ra01328b-f8.gif) | ||
| Fig. 8 Transformation of polysaccharides (1 g) by [MIMPS]H2PW12O40 (0.077 mmol, 0.5 mL water, 5.0 mL MIBK). The conversion and yield are given in the figure. | ||
Compared to other solid acid catalysts4,6–8,10,11,13,15–18,28–31 (Table S1, ESI†), IL HPA catalysts could promote the conversion of cellulose into glucose with a higher activity than other reported HPA catalysts such as H3PW12O40, Cs2.5H0.5PW12O40, Sn0.75PW12O40 and Cs2SnPW12O40. Compared with H5BW12O40, our catalysts show less activity in the conversion of cellulose. However, the usage of H5BW12O40 needs to be larger (1.4 mmol in 2 mL aqueous acidic solution) in order to confirm the conversion of cellulose with good results. The further research working on H5BW12O40 would be done including the introduction of some Lewis acid centers into H5BW12O40 molecules, loading H5BW12O40 on some Lewis supports, and using a micellar HPA system. In this way, we would obtain catalysts with strong Brønsted acidity, double acid sites, easy separation and a nanoreactor to enhance the conversion of cellulose into glucose with high selectivity.
4. Conclusions
Catalytic hydrolysis of cellulose into water-soluble products such as glucose and levulinic acid using IL HPA catalysts was investigated. The hydrolysis process was demonstrated to be efficient, and the conversion of cellulose and the yield of glucose reached 55.1% and 36.0%, respectively, at 140 °C for 5 h in a water–MIBK biphasic system. The high performance was attributed to the solubility of catalysts in water forming a homogeneous system and the Brønsted properties of HPAs. Furthermore, one-pot production of levulinic acid from cellulose and starch was realized by this catalyst with yields of 63.1% and 48.7% for reactions of 12 h and 5 h, respectively. The IL HPA catalyst could be completely separated and recovered from the reaction mixture. The retrieved catalyst could be reused six times without appreciable loss of performance.Acknowledgements
This work was supported by the National Natural Science Foundation of China (20871026, 51078066). And it was supported by analysis and testing foundation of Northeast Normal University and the major projects of Jilin Provincial Science and Technology Department (201105001).References
- G. W. Huber, S. Iborra and A. Corma, Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS
.
- P. L. Dhepe and A. Fukuoka, Cellulose conversion under heterogeneous catalysis, ChemSusChem, 2008, 1, 969–975 CrossRef CAS
.
- F. M. Jin and H. J. Enomoto, Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: chemistry of acid/base-catalysed and oxidation reactions, Energy Environ. Sci., 2011, 4, 382–397 CAS
.
- D. M. Lai, L. Deng, J. Li, B. Liao, Q. X. Guo and Y. Fu, Hydrolysis of cellulose into glucose by magnetic solid acid, ChemSusChem, 2011, 4, 55–58 CrossRef CAS
.
- M. J. Climent, A. Croma and S. Iborra, Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts, Green Chem., 2011, 13, 520–540 RSC
.
- A. Onda, T. Ochi and K. Yanagisawa, Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions, Top. Catal., 2009, 52, 801–807 CrossRef CAS
.
- A. Onda, T. Ochi and K. Yanagisawa, Selective hydrolysis of cellulose into glucose over solid acid catalysts, Green Chem., 2008, 10, 1033–1037 RSC
.
- J. F. Pang, A. Q. Wang, M. Y. Zheng and T. Zhang, Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures, Chem. Commun., 2010, 46, 6935–6937 RSC
.
- S. V. de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Catalytic production of levulinic acid from cellulose and other biomass-derived carbohydrates with sulfonated hyperbranched poly(arylene oxindole)s, Energy Environ. Sci., 2011, 4, 3601–3610 Search PubMed
.
- A. Takagaki, C. Tagusagawa and K. Domen, Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst, Chem. Commun., 2008, 5363–5365 RSC
.
- T. Okuhara, Water-tolerant solid acid catalysts, Chem. Rev., 2002, 102, 3641–3666 CrossRef CAS
.
- K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya and A. Satsuma, Effects of Brønsted and Lewis acidities on activity and selectivity of heteropolyacid-based catalysts for hydrolysis of cellobiose and cellulose, Green Chem., 2009, 11, 1627–1632 RSC
.
- J. Tian, J. H. Wang, S. Zhao, C. Y. Jiang, X. Zhang and X. H. Wang, Hydrolysis of cellulose by the heteropoly acid H3PW12O40, Cellulose, 2010, 17, 587–594 CrossRef CAS
.
- S. Zhao, M. X. Cheng, J. Z. Li, J. Tian and X. H. Wang, One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst, Chem. Commun., 2011, 47, 2176–2178 RSC
.
- J. Tian, C. Y. Fan, M. X. Cheng and X. H. Wang, Hydrolysis of cellulose over CsxH3−xPW12O40 (x = 1–3) aeteropoly acid catalysts, Chem. Eng. Technol., 2011, 34, 482–486 CrossRef CAS
.
- Y. Ogasawara, S. Itagaki, K. Yamaguchi and N. Mizuno, Saccharification of natural lignocellulose biomass and polysaccharides by highly negatively charged heteropolyacids in concentrated aqueous solution, ChemSusChem, 2011, 4, 519–525 CrossRef CAS
.
- F. Chambon, F. Rataboul, C. Pinel, A. Cabiac, E. Guillon and N. Essayem, Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid, Appl. Catal., B, 2011, 105, 171–181 CrossRef CAS
.
- J. Geboers, S. V. Vyver, K. Carpentier, P. Jacobs and B. Sels, Hydrolytic hydrogenation of cellulose with hydrotreated caesium salts of heteropoly acids and Ru/C, Green Chem., 2011, 13, 2167–2174 RSC
.
- S. V. de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, Recent advances in the catalytic conversion of cellulose, ChemCatChem, 2011, 3, 82–94 CrossRef
.
- H. Tadesse and R. Luque, Advances on biomass pretreatment using ionic liquids: An overview, Energy Environ. Sci., 2011 Search PubMed
, Advanced article.
- Y. Leng, J. Wang, D. R. Zhu, X. Q. Ren, H. Q. Ge and L. Shen, Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification, Angew. Chem., Int. Ed., 2009, 48, 168–171 CrossRef CAS
.
- C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure, Inorg. Chem., 1983, 22, 207–216 CrossRef CAS
.
- G. L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem., 1959, 31, 426–428 CrossRef CAS
.
- T. Okayasu, K. Saito, H. Nishide and M. T. W. Hearn, Poly(vinylsulfonic acid)-grafted solid catalysts: new materials for acid-catalysed organic synthetic reactions, Green Chem., 2010, 12, 1981–1989 RSC
.
- M. X. Cheng, T. Shi, H. Y. Guan, S. T. Wang, X. H. Wang and Z. J. Jiang, Clean production of glucose from polysaccharides using a micellar heteropolyacid as a heterogeneous catalyst, Appl. Catal., B, 2011, 107, 104–109 CrossRef CAS
.
- M. X. Cheng, T. Shi, S. T. Wang, H. Y. Guan, C. Y. Fan and X. H. Wang, Fabrication of micellar heteropolyacid catalysts for clean production of monosaccharides from polysaccharides, Catal. Commun., 2011, 12, 1483–1487 CrossRef CAS
.
- T. V. Stein, P. M. Grande, H. Kayser, F. Sibilla, W. Leitner and P. D. de Maria, From biomass to feedstock: one-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system, Green Chem., 2011, 13, 1772–1777 RSC
.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups, J. Am. Chem. Soc., 2008, 130, 12787–12793 CrossRef CAS
.
- Z. H. Zhang and Z. B. K. Zhao, Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid, Carbohydr. Res., 2009, 344, 2069–2072 CrossRef CAS
.
- R. Rinaldi, R. Palkovits and F. Schüth, Depolymerization of cellulose using solid catalysts in ionic liquids, Angew. Chem., Int. Ed., 2008, 47, 8047–8050 CrossRef CAS
.
- J. Zakzeski, R. J. H. Grisel, A.T. Smit and B. M. Weckhuysen, Solid acid-catalyzed cellulose hydrolysis monitored by in situ ATR-IR spectroscopy, ChemSusChem, 2012, 5, 430–437 CrossRef CAS
.
- Y. S. Qu, C. P. Huang, J. Zhang and B. H. Chen, Efficient dehydration of fructose to 5-hydroxymethylfurfural catalyzed by a recyclable sulfonated organic heteropolyacid salt, Bioresour. Technol., 2012, 106, 170–172 CrossRef CAS
.
- H. Wang, G. Gurau and R. D. Rogers, Ionic liquid processing of cellulose, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC
.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20198d/ |
| This journal is © The Royal Society of Chemistry 2012 |
