DOI:
10.1039/C2RA20840G
(Paper)
RSC Adv., 2012,
2, 6323-6328
Modulating the selectivity by switching sensing media: a bifunctional chemosensor selectivity for Cd2+ and Pb2+ in different aqueous solutions†
Received
2nd May 2012
, Accepted 10th May 2012
First published on 15th June 2012
Abstract
A novel bifunctional fluorescent probe RI, based on rhodamine with isatin, was designed and synthesized. Switching of the selectivity of RI between various metal ions was achieved by judicious choice of sensing media. RI gives a turn-on fluorometric and colorimetric signal toward Cd2+ in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Fluorescence imaging of Cd2+ in living cells was also obtained.
4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Fluorescence imaging of Cd2+ in living cells was also obtained.
Introduction
In the past few years, tremendous efforts have been devoted to the development of fluorescent and/or colorimetric chemosensors for selective and sensitive detection of heavy and transition metal (HTM) ions because of their diverse physiological roles in living systems and their environmental impact.1 HTM ions, in particular Cd2+ and Pb2+, have received focused attention due to their detrimental effects on public health. Recent studies indicate that human exposure to cadmium may cause lung, renal and prostate cancers and calcium metabolism disorders.2 Similarly, prolonged accumulation of lead in the body leads to many serious human health problems, including muscle paralysis, mental confusion, memory loss and anaemia.3 Therefore, development of high performance Cd2+ and Pb2+ probes is a very active field of research. A number of fluorescent and/or colorimetric chemosensors for each were reported in recent literature.4
It is quite often that Cd2+ and Pb2+ are found to coexist, such as in organic-rich soil profiles in the vicinity of a zinc smelter, in biowaste and in edible marine products including molluscs and crustaceans.5 We believe that if there was a single probe that could perform Cd2+ detection under one condition and Pb2+ detection under another, the cost for their measurement could potentially be greatly reduced, from the point of view of both the necessary reagents and laboratory effort. Such bifunctional fluorescent probes for other metal pairs, such as Pb2+/Hg2+, Au3+/Hg2+ and Fe3+/Cu2+ are already known.6 Our group also reported two examples of bifunctional fluorescent probes.7 One is for selective recognition of Cd2+ and Hg2+ and the other for selective recognition of Zn2+ and Cu2+. Modulation of metal ion selectivity was achieved via judicious choice of aqueous buffer solution in these two cases.
In this paper, we report a new rhodamine-based fluorescent probe, RI, whose selectivity to Cd2+ and Pb2+ is modulated by switching sensing media.
Results and discussion
Molecular design and synthesis
Herein, rhodamine B was chosen as the fluorophore, by virtue of its excellent photophysical properties, such as good photostability and long-wavelength absorption and emission.8 Besides, it is a convenient platform to construct colorimetric “naked eye” and/or fluorescence “off-on” probes, taking advantage of the opening of the spirolactam ring. Three examples of reported bifunctional probes were also based on the rhodamine scaffold.6a,6c,7b In this work, we incorporated an isatin moiety into the probe RI for metal coordination. Isatin and its derivatives are potent inhibitors of monoamine oxidase, caspase-3 and so on,9 while their potential for Cd2+ chelation was not examined. This is their first use in constructing a Cd2+ ligand.
Sensor RI was concisely synthesized from rhodamine B in two steps (Scheme 1). The structure of RI was verified by 1H NMR, 13C NMR and high resolution mass spectrometry (HRMS) (Fig. S1, ESI†).
 |
| | Scheme 1 Synthesis of RI. | |
The selectivity of RI toward Cd2+.
Initial screening of the metal binding ability of RI was carried out in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution. RI was nearly colorless and non-fluorescent in HEPES (10 mM, CH3OH–H2O, 4
6, v/v, pH 7.6) buffer solution. RI was nearly colorless and non-fluorescent in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution, due to its stable spirolactam form. Upon addition of Cd2+ to a solution of RI, the solution instantaneously changed from nearly colorless to pink, and significant enhancement of fluorescence with an emission maximum at 581 nm was observed. However, the other ions, including Pb2+, produced only minor changes in the fluorescence spectra (Fig. 1). Therefore, RI was a highly selective fluorescence “off-on” probe for Cd2+ in HEPES (10 mM, CH3OH–H2O, 4
6, v/v, pH 7.6) buffer solution, due to its stable spirolactam form. Upon addition of Cd2+ to a solution of RI, the solution instantaneously changed from nearly colorless to pink, and significant enhancement of fluorescence with an emission maximum at 581 nm was observed. However, the other ions, including Pb2+, produced only minor changes in the fluorescence spectra (Fig. 1). Therefore, RI was a highly selective fluorescence “off-on” probe for Cd2+ in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution.
6, v/v, pH 7.6) buffer solution.
The selectivity of RI toward Pb2+.
As it has been shown that the selectivity of the chemosensor could be profoundly affected by switching various parameters of the sensing media, such as the solvent, pH, buffer, ionic strength and so on,10 further investigations of the selectivity of RI in alternative systems were also carried out.
The solution of RI in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution was also nearly colorless and did not exhibit apparent absorption above 500 nm, due to the formation of the stable spirolactam. Addition of Pb2+ to a solution of RI led to an obvious absorption enhancement at 563 nm, along with an obvious color change from colorless to purple (Fig. 2). However, no enhancement of fluorescence was observed, and we supposed that the fluorescence quenching caused by Pb2+ belonged to static quenching.11 In this solvent system, it is interesting to see that all the other metal ions including Cd2+ did not lead to the same absorption enhancement under the same conditions. The selectivity was again confirmed via competition experiments (Fig. S5, ESI†). The above results suggest that RI can serve as a highly selective “naked eye” probe for Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
4, v/v, pH 7.6) buffer solution was also nearly colorless and did not exhibit apparent absorption above 500 nm, due to the formation of the stable spirolactam. Addition of Pb2+ to a solution of RI led to an obvious absorption enhancement at 563 nm, along with an obvious color change from colorless to purple (Fig. 2). However, no enhancement of fluorescence was observed, and we supposed that the fluorescence quenching caused by Pb2+ belonged to static quenching.11 In this solvent system, it is interesting to see that all the other metal ions including Cd2+ did not lead to the same absorption enhancement under the same conditions. The selectivity was again confirmed via competition experiments (Fig. S5, ESI†). The above results suggest that RI can serve as a highly selective “naked eye” probe for Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution.
4, v/v, pH 7.6) buffer solution.
 |
| | Fig. 2 UV-vis absorption spectra of RI (10 μM) in the presence of 160 μM solutions of various metal ions, such as Fe2+, Zn2+, Cd2+, Pb2+, Ba2+, Hg2+, Mg2+, Co2+, Ni2+, Ca2+, Mn2+, Cu2+, Fe3+, Cr3+, Li+, Ag+ and K+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. Inset: the photograph shows the color change of RI (10 μM) in the presence of 160 μM solutions of various metal ions; from left to right: RI, Cd2+, Hg2+, Zn2+, Pb2+, Cu2+, Ni2+, Fe3+, Co+, other mixtures. 4, v/v, pH 7.6) buffer solution. Inset: the photograph shows the color change of RI (10 μM) in the presence of 160 μM solutions of various metal ions; from left to right: RI, Cd2+, Hg2+, Zn2+, Pb2+, Cu2+, Ni2+, Fe3+, Co+, other mixtures. | |
Cd2+-titration and Pb2+-titration.
The UV-vis and fluorescence titrations were carried out by gradual addition of various amounts of Cd2+ and Pb2+, respectively (Fig. 3; Fig. S3, ESI†). A nearly linear relationship was obtained between the fluorescence intensity of sensor RI and the low concentration of Cd2+ in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution, respectively. The results indicate that RI is sensitive towards Cd2+ and Pb2+ in the corresponding sensing media.
4, v/v, pH 7.6) buffer solution, respectively. The results indicate that RI is sensitive towards Cd2+ and Pb2+ in the corresponding sensing media.
 |
| | Fig. 3 Fluorescence spectra (a) and UV-vis absorption spectra (b, c, d) of RI (10 μM) upon addition of (a) Cd2+ (10 μM∼150 μM), (b) Pb2+ (10 μM∼40 μM) in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and (c) Cd2+ (5 μM∼30 μM), (d) Pb2+ (5 μM∼260 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6 6, v/v, pH 7.6) buffer solution and (c) Cd2+ (5 μM∼30 μM), (d) Pb2+ (5 μM∼260 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. 4, v/v, pH 7.6) buffer solution. | |
The proposed binding modes of RI with Cd2+ and Pb2+
To further study the specific binding model, the 1H NMR titration experiments were measured. As shown in Fig. S6,† a set of new peaks appeared with the addition of Cd2+ into the solution of RI in CD3CN. The apparent downshift of H1 and H2 in the presence of Cd2+ suggests the formation of the ring-opened form of rhodamine.12 The downshift of H3 indicates that the oxygen atom of the amide group participates in the binding. In addition, the H4 upshift from 7.98 to 7.89 is due to the “O” on the hydrazide group participating in the binding. Taken together, based on the 1H NMR titration, the coordinating properties of Cd2+ and the Job plot, a possible binding mode between RI and Cd2+ is shown in Scheme 2. Since Pb2+ belongs to static quenching, the 1H NMR titration experiment failed. We proposed a possible binding mode between RI and Pb2+ similar to Cd2+. The energy-minimized structures of RI with both Cd2+ and Pb2+ were calculated by the Gaussian program (Fig. 5).
 |
| | Fig. 5 Energy-minimized structures of (a) RI, (b) RI with Cd2+, (c) RI with Pb2+ and (d) the overlap of (b) and (c) were calculated by the B3LYP method with the 6-311G basis set (Gaussian 09 programme). | |
 |
| | Scheme 2 Proposed binding modes of RI with (a) Cd2+ and (b) Pb2+. | |
The influences of buffer and solvent on the selectivity of RI
The influences of buffer on the selectivity of RI were determined. As shown in Fig. 6(a, c) and Fig. 7(a, c), the response of RI to Cd2+ is barely affected by the concentration of HEPES; however, it is significantly affected by the concentration of the Tris buffer. As shown in Fig. 6(b, d) and Fig. 7(b, d), the response of RI to Pb2+ is barely affected by the concentration of neither the HEPES nor the Tris buffer. It is known that Tris contains hydroxy and amino groups, which could chelate different metal ions with varied affinities. The above results indicate that Tris competes with the probe for the binding of Cd2+ but not Pb2+. The affinity between RI and Cd2+ was totally overwhelmed when a bolus concentration of Tris buffer was used.
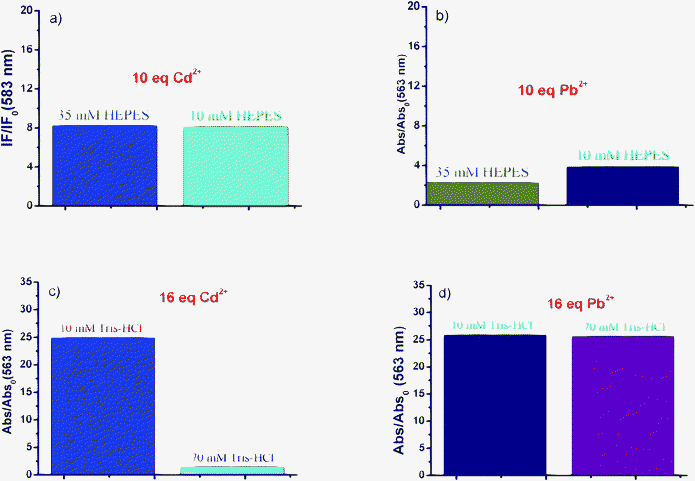 |
| | Fig. 7 The ratiometric (a) fluorescence and (b) UV-vis absorption changes of RI (10 μM) in the presence of (a) Cd2+ (100 μM) and (b) Pb2+ (100 μM) in 10 mM or 35 mM HEPES (CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution. The ratiometric UV-vis absorption changes of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6 6, v/v, pH 7.6) buffer solution. The ratiometric UV-vis absorption changes of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. 4, v/v, pH 7.6) buffer solution. | |
 |
| | Fig. 8 (a) Bright-field transmission image of Hela cells pre-incubated with sensor RI (10 μM) for 30 min, washed three times, and then treated with Cd2+ (6.0 equiv); (b) fluorescence image of (a); (c) bright-field transmission image of Hela cells pre-incubated with sensor RI (10 μM) for 30 min, washed three times; (d) fluorescence image of (c). | |
Conclusions
In conclusion, we have developed a new bifunctional fluorescent probe RI based on rhodamine, with a novel receptor. We modulated the selectivity of RI to various metal ions by switching the sensing media. RI gives a turn-on fluorometric and colorimetric signal toward Cd2+ in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Furthermore, we have demonstrated that the sensor RI was applicable for Cd2+ imaging in the living Hela cells.
4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Furthermore, we have demonstrated that the sensor RI was applicable for Cd2+ imaging in the living Hela cells.
Experimental
Materials and measurements
Unless otherwise mentioned, all the reagents were of analytical grade. 1H NMR and 13C NMR spectra were measured on a Bruker AM-400 spectrometer, with chemical shifts reported in ppm (in CDCl3, TMS as internal standard). Mass spectra were obtained with a HP 5989A spectrometer. All pH measurements were made with a Sartorius basic pH-Meter PB-20. Absorption spectra were determined on a Varian Cary 100 Spectrophotometer. Fluorescence spectra were determined on a Varian Cary Eclipse. HPLC traces were determined on a HP-1100 spectrometer.
Synthesis of probe RI
Rhodamine B hydrazide (1).
In this work, 1 was synthesized according to ref. 14. In a 50 mL flask, rhodamine B (0.48 g, 1.0 mmol) was dissolved in 12 mL ethanol. 1.2 mL (excess) hydrazine hydrate (85%) was then added. After the addition, the mixture was heated under reflux for 2 h. The solution changed from dark purple to light orange and became clear. Then the mixture was cooled and the solvent removed under reduced pressure. 1 M HCl (about 20 mL) was added to the solid in the flask to generate a clear red solution. After that, 1 M NaOH (about 28 mL) was added slowly with stirring until the pH of the solution reached 9∼10. The resulting precipitate was filtered and washed 3 times with 6 mL water, and then dried in air. The product was then chromatographed on silica gel using CH2Cl2–CH3OH (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent, to afford 0.37 g (81%) 1 as a pink solid.
1, v/v) as the eluent, to afford 0.37 g (81%) 1 as a pink solid.
RI.
1 (0.64 mmol, 300 mg) was dissolved in 10 mL absolute methanol, then 2,3-indolinedione (0.76 mmol, 112 mg) was added. Then the mixture was heated under reflux and stirred for 6 h. After that, the solvent was removed under vacuum to give an orange solid. The crude product was then chromatographed on silica gel using CH2Cl2–CH3OH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, /v) as the eluent, to afford 242 mg (63%) RI as an orange solid. 1H NMR (CDCl3, 400 MHz): δ 1.15 (t, J = 7.0 Hz, 12H), 3.28–3.34 (m, 8H), 6.25 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 2H), 6.41 (d, J = 2.0 Hz, 2H), 6.61 (d, J = 8.8 Hz, 2H), 6.76 (d, J = 8.0 Hz, 1H), 7.00 (t, J = 7.8 Hz, 1H), 7.19–7.27 (m, 3H), 7.50–7.57 (m, 2H), 8.02 (d, J = 6.8 Hz, 1H), 8.37 (s, 1H). 13C NMR (CDCl3, 100 MHz): δ 12.69, 44.28, 68.56, 98.12, 106.09, 108.01, 111.04, 118.08, 122.59, 123.98, 124.24, 127.92, 128.10, 128.43, 128.76, 132.93, 133.59, 143.82, 148.76, 151.64, 152.58, 153.45, 160.72, 165.14. HRMS (ES+): Calcd for ([M+H])+, 586.2818, found, 586.2819.
1, /v) as the eluent, to afford 242 mg (63%) RI as an orange solid. 1H NMR (CDCl3, 400 MHz): δ 1.15 (t, J = 7.0 Hz, 12H), 3.28–3.34 (m, 8H), 6.25 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 2H), 6.41 (d, J = 2.0 Hz, 2H), 6.61 (d, J = 8.8 Hz, 2H), 6.76 (d, J = 8.0 Hz, 1H), 7.00 (t, J = 7.8 Hz, 1H), 7.19–7.27 (m, 3H), 7.50–7.57 (m, 2H), 8.02 (d, J = 6.8 Hz, 1H), 8.37 (s, 1H). 13C NMR (CDCl3, 100 MHz): δ 12.69, 44.28, 68.56, 98.12, 106.09, 108.01, 111.04, 118.08, 122.59, 123.98, 124.24, 127.92, 128.10, 128.43, 128.76, 132.93, 133.59, 143.82, 148.76, 151.64, 152.58, 153.45, 160.72, 165.14. HRMS (ES+): Calcd for ([M+H])+, 586.2818, found, 586.2819.
Acknowledgements
We are grateful for the financial support from National Basic Research Program of China (973 Program, 2010CB126100), the China 111 Project (Grant B07023), the Key New Drug Creation and Manufacturing Program (Grant 2009ZX09103-102) and the Shanghai Leading Academic Discipline Project (B507). We also thank Professor Youjun Yang of ECUST for discussions and suggestions.
References
-
(a) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS;
(b) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS;
(c) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS;
(d) J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416 RSC.
-
(a) A. M. Jane, M. S. Matin, T. Amy, M. H. John and A. N. Polly, J. Natl. Cancer Inst., 2006, 98, 869 CrossRef;
(b) A. Akesson, B. Julin and A. Wolk, Cancer Res., 2008, 68, 6435 CrossRef CAS.
- H. Son, H. Y. Lee, J. M. Lim, D. Kang, W. S. Han, S. S. Lee and J. H. Jung, Chem.–Eur. J., 2010, 16, 11549 CrossRef CAS.
-
(a) M. Choi, M. Kim, K. D. Lee, K.-N. Han, I.-A. Yoon, H.-J. Chung and J. Yoon, Org. Lett., 2001, 3, 3455 CrossRef CAS;
(b) X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500 CrossRef CAS;
(c) G. M. Cockrell, G. Zhang, D. G. VanDerveer, R. P. Thummel and R. D. Hancock, J. Am. Chem. Soc., 2008, 130, 1420 CrossRef CAS;
(d) T. Cheng, Y. Xu, S. Zhang, W. Zhu, X. Qian and L. Duan, J. Am. Chem. Soc., 2008, 130, 16160 CrossRef CAS;
(e) M. Taki, M. Desaki, A. Ojida, S. Iyoshi, T. Hirayama, I. Hamachi and Y. Yamamoto, J. Am. Chem. Soc., 2008, 130, 12564 CrossRef CAS;
(f) X. L. Tang, X. H. Peng, W. Dou, J. Mao, J. R. Zheng, W. W. Qin, W. S. Liu, J. Chang and X. J. Yao, Org. Lett., 2008, 10, 3653 CrossRef CAS;
(g) L. Xue, C. Liu and H. Jiang, Org. Lett., 2009, 11, 1655 CrossRef CAS;
(h) Z. Xu, K. -H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef CAS;
(i) Z. Liu, C. Zhang, W. He, Z. Yang, X. Gao and Z. Guo, Chem. Commun., 2010, 46, 6138 RSC;
(j) A. K. Mahapatra, J. Roy and P. Sahoo, Tetrahedron Lett., 2011, 52, 2965 CrossRef CAS;
(k) J. Y. Kwon, Y. J. Jang, Y. J. Lee, K. M. Kim, M. S. Seo, W. Nam and J. Yoon, J. Am. Chem. Soc., 2005, 127, 10107 CrossRef CAS;
(l) K. Kavallieratos, J. M. Rosenberg, W. Z. Chen and T. Ren, J. Am. Chem. Soc., 2005, 127, 6514 CrossRef CAS;
(m) Q. He, E. W. Miller, A. P. Wong and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9316 CrossRef CAS;
(n) L. J. Ma, Y. F. Liu and Y. Wu, Chem. Commun., 2006, 2702 RSC;
(o) E. Roussakis, S. A. Pergantis and H. E. Katerinopoulos, Chem. Commun., 2008, 6221 RSC;
(p) F. Zapata, A. Caballero, A. Espinosa, A. Tarraga and P. Molina, Org. Lett., 2008, 10, 41 CrossRef CAS;
(q) H. Y. Lee, D. R. Bae, J. C. Park, H. Song, W. S. Han and J. H. Jung, Angew. Chem., Int. Ed., 2009, 48, 1239 CrossRef CAS;
(r) L. Marbella, B. S. Mitasev and P. Basu, Angew. Chem., Int. Ed., 2009, 48, 3996 CrossRef CAS;
(s) Y. Xiang, A. Tong and Y. Lu, J. Am. Chem. Soc., 2009, 131, 15352 CrossRef CAS;
(t) E. Ranyuk, C. M. Douaihy, A. Bessmertnykh, F. Denat, A. Averin, I. Beletskaya and R. Guilard, Org. Lett., 2008, 11, 987 CrossRef;
(u) T. Li, S. Dong and E. Wang, J. Am. Chem. Soc., 2010, 132, 13156 CrossRef CAS;
(v) R. Zhou, B. Li, N. Wu, G. Gao, J. You and J. Lan, Chem. Commun., 2011, 47, 6668 RSC.
-
(a) M. L. Svendsen, E. Steinnes and H. A. Blom, Chem. Speciation Bioavailability, 2011, 23, 189 CrossRef CAS;
(b) A. Veeken and B. Hamelers, Sci. Total Environ., 2002, 300, 87 CrossRef CAS;
(c) J. M. Llobet, G. Falco, C. Casas, A. Teixido and J. L. Domingo, J. Agric. Food Chem., 2003, 51, 838 CrossRef CAS.
-
(a) Z. Q. Hu, C. Lin, X. M. Wang, L. Ding, C. L. Cui, S. F. Liu and H. Y. Lu, Chem. Commun., 2010, 46, 3765 RSC;
(b) M. Dong, Y. W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310 CrossRef CAS;
(c) L. F. Zhang, J. L. Zhao, X. Zeng, L. Mu, X. K. Jiang, M. Deng, J. X. Zhang and G. Wei, Sens. Actuators, B, 2011, 160, 662 CrossRef CAS.
-
(a) T. Cheng, T. Wang, W. Zhu, Y. Yang, B. Zeng, Y. Xu and X. Qian, Chem. Commun., 2011, 47, 3915 RSC;
(b) L. Xu, Y. Xu, W. Zhu, B. Zeng, C. Yang, B. Wu and X. Qian, Org. Biomol. Chem., 2011, 9, 8284 RSC.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
-
(a) C. I. M-King, J. J. Bergh and J. P. Petzer, Bioorg. Med. Chem., 2011, 19, 261 CrossRef;
(b) Y. Jiang and T. V. Hansen, Bioorg. Med. Chem. Lett., 2011, 21, 1626 CrossRef CAS.
-
(a) J. Han and K. Burgess, Chem. Rev., 2010, 110, 2709 CrossRef CAS;
(b) B. Tang, F. Yu, P. Li, L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016 CrossRef CAS.
- M. Y. Chae, J. Yoon and A. W. Czarnik, J. Mol. Recognit., 1996, 9, 297 CrossRef CAS.
- J. Huang, Y. Xu and X. Qian, J. Org. Chem., 2009, 74, 2167 CrossRef CAS.
-
(a) Y. Zhou, X. Y. You, Y. Fang, J. Y. Li, K. Liu and C. Yao, Org. Biomol. Chem., 2010, 8, 4819 RSC;
(b) K. Huang, H. Yang, Z. Zhou, M. Yu, F. Li, X. Gao, T. Yi and C. Huang, Org. Lett., 2008, 10, 2557 CrossRef CAS.
- Y. Xiang, A. Tong, P. Jin and Y. Ju, Org. Lett., 2006, 8, 2863 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and spectroscopic data. See DOI: 10.1039/c2ra20840g |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Fluorescence imaging of Cd2+ in living cells was also obtained.
4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Fluorescence imaging of Cd2+ in living cells was also obtained.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution. RI was nearly colorless and non-fluorescent in HEPES (10 mM, CH3OH–H2O, 4
6, v/v, pH 7.6) buffer solution. RI was nearly colorless and non-fluorescent in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution, due to its stable spirolactam form. Upon addition of Cd2+ to a solution of RI, the solution instantaneously changed from nearly colorless to pink, and significant enhancement of fluorescence with an emission maximum at 581 nm was observed. However, the other ions, including Pb2+, produced only minor changes in the fluorescence spectra (Fig. 1). Therefore, RI was a highly selective fluorescence “off-on” probe for Cd2+ in HEPES (10 mM, CH3OH–H2O, 4
6, v/v, pH 7.6) buffer solution, due to its stable spirolactam form. Upon addition of Cd2+ to a solution of RI, the solution instantaneously changed from nearly colorless to pink, and significant enhancement of fluorescence with an emission maximum at 581 nm was observed. However, the other ions, including Pb2+, produced only minor changes in the fluorescence spectra (Fig. 1). Therefore, RI was a highly selective fluorescence “off-on” probe for Cd2+ in HEPES (10 mM, CH3OH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution.
6, v/v, pH 7.6) buffer solution.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution.
6, v/v, pH 7.6) buffer solution.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution was also nearly colorless and did not exhibit apparent absorption above 500 nm, due to the formation of the stable spirolactam. Addition of Pb2+ to a solution of RI led to an obvious absorption enhancement at 563 nm, along with an obvious color change from colorless to purple (Fig. 2). However, no enhancement of fluorescence was observed, and we supposed that the fluorescence quenching caused by Pb2+ belonged to static quenching.11 In this solvent system, it is interesting to see that all the other metal ions including Cd2+ did not lead to the same absorption enhancement under the same conditions. The selectivity was again confirmed via competition experiments (Fig. S5, ESI†). The above results suggest that RI can serve as a highly selective “naked eye” probe for Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
4, v/v, pH 7.6) buffer solution was also nearly colorless and did not exhibit apparent absorption above 500 nm, due to the formation of the stable spirolactam. Addition of Pb2+ to a solution of RI led to an obvious absorption enhancement at 563 nm, along with an obvious color change from colorless to purple (Fig. 2). However, no enhancement of fluorescence was observed, and we supposed that the fluorescence quenching caused by Pb2+ belonged to static quenching.11 In this solvent system, it is interesting to see that all the other metal ions including Cd2+ did not lead to the same absorption enhancement under the same conditions. The selectivity was again confirmed via competition experiments (Fig. S5, ESI†). The above results suggest that RI can serve as a highly selective “naked eye” probe for Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution.
4, v/v, pH 7.6) buffer solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. Inset: the photograph shows the color change of RI (10 μM) in the presence of 160 μM solutions of various metal ions; from left to right: RI, Cd2+, Hg2+, Zn2+, Pb2+, Cu2+, Ni2+, Fe3+, Co+, other mixtures.
4, v/v, pH 7.6) buffer solution. Inset: the photograph shows the color change of RI (10 μM) in the presence of 160 μM solutions of various metal ions; from left to right: RI, Cd2+, Hg2+, Zn2+, Pb2+, Cu2+, Ni2+, Fe3+, Co+, other mixtures.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution, respectively. The results indicate that RI is sensitive towards Cd2+ and Pb2+ in the corresponding sensing media.
4, v/v, pH 7.6) buffer solution, respectively. The results indicate that RI is sensitive towards Cd2+ and Pb2+ in the corresponding sensing media.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and (c) Cd2+ (5 μM∼30 μM), (d) Pb2+ (5 μM∼260 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and (c) Cd2+ (5 μM∼30 μM), (d) Pb2+ (5 μM∼260 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution.
4, v/v, pH 7.6) buffer solution.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with both Cd2+ and Pb2+ (Fig. 4). The association constants between RI and Cd2+ and Pb2+ in different systems were determined, as shown in Table 1.
1 stoichiometric complex with both Cd2+ and Pb2+ (Fig. 4). The association constants between RI and Cd2+ and Pb2+ in different systems were determined, as shown in Table 1.
![Job plot of (a) RI and Cd2+ ([RI] + [Cd2+] = 80 μM) in HEPES (10 mM, CH3OH–H2O, 4 : 6, v/v, pH 7.6) and (b) RI and Pb2+ ([RI] + [Pb2+] = 80 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6 : 4, v/v, pH 7.6) buffer solutions.](/image/article/2012/RA/c2ra20840g/c2ra20840g-f4.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) and (b) RI and Pb2+ ([RI] + [Pb2+] = 80 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) and (b) RI and Pb2+ ([RI] + [Pb2+] = 80 μM) in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solutions.
4, v/v, pH 7.6) buffer solutions.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution. UV-vis absorption spectra of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution. UV-vis absorption spectra of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution.
4, v/v, pH 7.6) buffer solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution. The ratiometric UV-vis absorption changes of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution. The ratiometric UV-vis absorption changes of RI (10 μM) in the presence of (c) Cd2+ (160 μM) and (d) Pb2+ (160 μM) in 10 mM or 70 mM Tris-HCl (CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution.
4, v/v, pH 7.6) buffer solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6
6, v/v, pH 7.6) buffer solution and a turn-on colorimetric signal toward Pb2+ in Tris-HCl (70 mM, CH3OH–H2O, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Furthermore, we have demonstrated that the sensor RI was applicable for Cd2+ imaging in the living Hela cells.
4, v/v, pH 7.6) buffer solution. To the best of our knowledge, this is the first example of a chemosensor based on a small molecule that can selectively recognize both Cd2+ and Pb2+ in different sensing systems. Furthermore, we have demonstrated that the sensor RI was applicable for Cd2+ imaging in the living Hela cells.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent, to afford 0.37 g (81%) 1 as a pink solid.
1, v/v) as the eluent, to afford 0.37 g (81%) 1 as a pink solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, /v) as the eluent, to afford 242 mg (63%) RI as an orange solid. 1H NMR (CDCl3, 400 MHz): δ 1.15 (t, J = 7.0 Hz, 12H), 3.28–3.34 (m, 8H), 6.25 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 2H), 6.41 (d, J = 2.0 Hz, 2H), 6.61 (d, J = 8.8 Hz, 2H), 6.76 (d, J = 8.0 Hz, 1H), 7.00 (t, J = 7.8 Hz, 1H), 7.19–7.27 (m, 3H), 7.50–7.57 (m, 2H), 8.02 (d, J = 6.8 Hz, 1H), 8.37 (s, 1H). 13C NMR (CDCl3, 100 MHz): δ 12.69, 44.28, 68.56, 98.12, 106.09, 108.01, 111.04, 118.08, 122.59, 123.98, 124.24, 127.92, 128.10, 128.43, 128.76, 132.93, 133.59, 143.82, 148.76, 151.64, 152.58, 153.45, 160.72, 165.14. HRMS (ES+): Calcd for ([M+H])+, 586.2818, found, 586.2819.
1, /v) as the eluent, to afford 242 mg (63%) RI as an orange solid. 1H NMR (CDCl3, 400 MHz): δ 1.15 (t, J = 7.0 Hz, 12H), 3.28–3.34 (m, 8H), 6.25 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 2H), 6.41 (d, J = 2.0 Hz, 2H), 6.61 (d, J = 8.8 Hz, 2H), 6.76 (d, J = 8.0 Hz, 1H), 7.00 (t, J = 7.8 Hz, 1H), 7.19–7.27 (m, 3H), 7.50–7.57 (m, 2H), 8.02 (d, J = 6.8 Hz, 1H), 8.37 (s, 1H). 13C NMR (CDCl3, 100 MHz): δ 12.69, 44.28, 68.56, 98.12, 106.09, 108.01, 111.04, 118.08, 122.59, 123.98, 124.24, 127.92, 128.10, 128.43, 128.76, 132.93, 133.59, 143.82, 148.76, 151.64, 152.58, 153.45, 160.72, 165.14. HRMS (ES+): Calcd for ([M+H])+, 586.2818, found, 586.2819.
