A single molecule multi analyte chemosensor differentiates among Zn2+, Pb2+ and Hg2+: modulation of selectivity by tuning of solvents †‡
Joydev
Hatai
,
Suman
Pal
,
Gregor P.
Jose
,
Tapas
Sengupta
and
Subhajit
Bandyopadhyay
*
Indian Institute of Science Education and Research (IISER) – Kolkata, BCKV Main Campus PO, Mohanpur, Nadia, WB 741252, India. E-mail: sb1@iiserkol.ac.in
First published on 11th July 2012
Abstract
A chemosensor detects and differentiates among Hg2+, Pb2+ and Zn2+ with fluorescence enhancement at different wavelengths under different solvent conditions. In methanol, Hg2+ and Pb2+ displace Zn2+ from the [Zn2+-sensor] complex displaying ratiometric behaviour. In water, the chemosensor recognises only Hg2+ with fluorescence enhancement, and offers intracellular detection of Hg2+.
Fluoroscence sensing is a rapidly evolving field of research.1 Sensing multiple analytes with a single chemosensor is a challenging task. It involves eliciting different responses by the molecule in the presence of different analytes. Such a molecule would involve either multiple binding sites giving rise to different binding modes, or multiple signal transducing units (e.g., chromophores/fluorophores/redox active moieties), multiple read-out modes or any combination of the above. Chemosensors with selectivity for two analytes have received much attention in recent years. There are reports of two-ion sensors that detect Ba2+–Hg2+,2a Hg2+–Fe2+,2b Al3+–Zn2+,2c Zn2+–Cd2+,2d Cu2+–Zn2+,2e Hg2+–Pb2+,2f and Hg2+–Cr3+.2g The above examples, for brevity, are non-exclusive. Selective detection of three analytes with a chemosensor having only one kind of fluorophore is rare in the literature. Such a chemosensor is highly coveted in terms of convenience and economy. Three analyte molecular receptors have been used for the construction of molecular logic gates where an emission output rather than the differentiation of response by multiple analyte is important.3 The Zn2+ ion is present in several metalloenzymes and is indispensable for proper cellular functions. It plays a crucial role in normal functioning of the central nervous system.4 Zinc deficiency causes impaired liver, kidney and neural functions,5 whereas an excess intake suppresses absorption of copper and iron, leading to other secondary problems.6 Pb2+ and Hg2+, on the other hand, are toxic even at a very low levels and cause serious damage to the skin, nervous and renal systems.7 The US-EPA guidelines allow a maximum acceptable concentration of 0.015 and 0.002 ppm of inorganic lead and mercury respectively in drinking water.7
In this communication, we report a coumarin based fluorescent chemosensor 1 on a thiocarbamate scaffold that displays different selectivity towards Hg2+, Pb2+ and Zn2+ ions under different conditions. In non-aqueous media, chemosensor 1 detects Hg2+, and Pb2+ with enhancement of the ligand fluorescence band at 410 nm. With Zn2+, the chemosensor displays ratiometric fluorescence behaviour at 410 and 495 nm. Both Hg2+ and Pb2+ can displace Zn2+ from the [Zn2+·1] complex and demonstrate ratiometric behaviour. In aqueous media, the chemosensor selectively recognises only Hg2+ with a remarkable fluorescence enhancement at nmol L−1 concentrations. The S donor atom in the photo-induced electron transfer (PET) process ensures its activity even at low pH.8 In aqueous media, the sensor also detects intracellular Hg2+.
The bipodal chemosensor 1 consists of two thiocarbamate moieties attached to coumarin units via ethylenediamine spacers (Fig. 1). It was synthesized from bis-bromomethyl salicylaldehyde and ethylenediamine-coumarin conjugate in the presence of CS2 under aqueous conditions (Scheme S1, SI‡). The compound was characterised by 1D and 2D NMR and other spectroscopic methods. The sensing behaviour of 1 was investigated by UV-vis and fluorescence measurements with several metal ions i.e., Na+, K+, Al3+, Ca2+, Cd2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Li+, Ba2+, Ag+ and Hg2+ in CH3OH and H2O with 1% DMSO as a co-solvent in the presence of HEPES buffered at pH 7.0. The absorption spectrum of 1 (10 μmol L−1) in MeOH showed absorption bands at 280 nm and 335 nm. Systematic titration of sensor 1 with increasing concentrations of Zn2+ revealed a continuous intensity increase of the absorption band at 280 nm with a new charge transfer transition band at 400 nm (Fig. S1, SI‡). In contrast, titration of 1 with one equivalent of Hg2+ resulted in a small decrease in the absorption at 280 nm with a 12 nm bathochromic shift whereas the absorption at 335 nm increased in intensity (Fig. S2, SI‡). Under the same conditions with increasing concentrations of Pb2+ a decrease in the absorption intensity with a bathochromic shift to 290 nm (Δλ = 10 nm) was observed (Fig. S3, SI‡). Interestingly, in aqueous media, 1 showed ratiometric behaviour with Hg2+. Upon titration of 1 (10 μmol L−1) with Hg2+ (0–30 μM) the absorption at 400 nm gradually decreased in intensity, whereas the absorption at 280 nm went up with an isosbestic point at 350 nm (Fig. S4, SI‡).
 | ||
| Fig. 1 Structure of chemosensor 1. | ||
The emission spectrum of 1 (λex = 320 nm, slit 5/5 nm) in MeOH showed a characteristic fluorescence band (quantum yield, Φf = 0.116) at 410 nm (Fig. 2A). Upon addition of the above perchlorate salts, 1 with Zn2+ showed a significant decrease in the 410 nm emission band. A new red shifted (85 nm) intense emission band centered at 495 nm (Φf = 0.50) was also observed with an isoemission point at 450 nm, indicating Zn2+ selective ratiometric behaviour (Fig. 2B). The new band at 495 nm in the presence of Zn2+ is most likely an ICT based optical response.2c
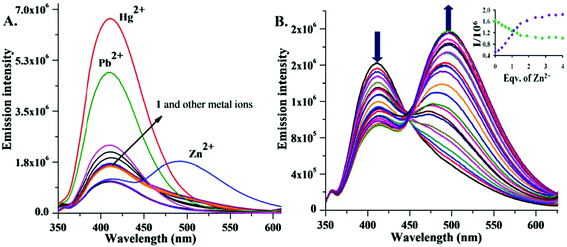 | ||
Fig. 2 (A) Change in fluorescence intensity of 1 (10 μmol L−1) in MeOH/DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with different cations (20 μM) in the presence of HEPES buffer at pH 7.0. (B) Fluorescence spectra of 1 (10 μmol L−1) upon addition of Zn2+ (0 to 40 μmol L−1) in MeOH/DMSO (99 1, v/v) with different cations (20 μM) in the presence of HEPES buffer at pH 7.0. (B) Fluorescence spectra of 1 (10 μmol L−1) upon addition of Zn2+ (0 to 40 μmol L−1) in MeOH/DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Inset: Zn2+ titration profile at 410 and 492 nm. 1, v/v). Inset: Zn2+ titration profile at 410 and 492 nm. | ||
Furthermore, under the same conditions, Hg2+ and Pb2+ only enhanced the intensity (Φf = 0.58 and 0.39) of the 410 nm fluorescence band, suggesting 1 as an efficient Hg2+, Pb2+ selective fluorescence turn-ON sensor based on the PET mechanism (Fig. 2A). With other metal ions, a small fluorescence enhancement (Cd2+, Ag+, Cu2+) or quenching (Ni2+, Mn2+, Co2+, Fe2+, Fe3+) was observed (Fig. 2A and S5, SI‡).
The stoichiometry of the Zn2+·1 association was determined by a Job's plot from the UV-vis absorption data (Fig. S7, SI‡).9 The plot revealed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the Zn2+ ions associating with each molecule of 1. A peak at m/z 907 in ESI-MS further supported the 2
1 ratio of the Zn2+ ions associating with each molecule of 1. A peak at m/z 907 in ESI-MS further supported the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (Fig. S20, SI‡). The binding constant for the complex of Zn2+ with 1 was calculated using the method of Lehrer and Chipman from the fluorescence data (Fig. S11, SI‡).10 The stoichiometry of the Zn2+·1 association thus obtained was 2.08, which is again in perfect agreement with the 2
1 binding stoichiometry (Fig. S20, SI‡). The binding constant for the complex of Zn2+ with 1 was calculated using the method of Lehrer and Chipman from the fluorescence data (Fig. S11, SI‡).10 The stoichiometry of the Zn2+·1 association thus obtained was 2.08, which is again in perfect agreement with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio obtained earlier. From the intercept of the best fitting straight line, an association constant of 7.07 × 104 mol2 L−2 was obtained. The analytical detection limit of Zn2+ determined following the reported method11 (Fig. S15, SI‡) was 0.58 μmol L−1.
1 ratio obtained earlier. From the intercept of the best fitting straight line, an association constant of 7.07 × 104 mol2 L−2 was obtained. The analytical detection limit of Zn2+ determined following the reported method11 (Fig. S15, SI‡) was 0.58 μmol L−1.
Interestingly, the addition of Hg2+ and Pb2+ to a solution of the Zn2+·1 complex results in a shift in the fluorescence emission from green to violet. Thus, the Zn2+·1 ensemble can be used to detect Hg2+ and Pb2+via the displacement of the Zn2+ ion based on the higher affinity of 1 for these two ions. Upon addition of an increasing concentration of Hg2+ and Pb2+, the emission maximum of the Zn2+·1 complex at 495 nm gradually decreased with an increase of the band at 410 nm (Fig. 3A and 3B) with the formation of clear isoemission points (at 480 nm for Hg2+, and 465 nm for Pb2+). Moreover, the replacement of Zn2+ by Hg2+ and Pb2+ has been confirmed by 1H NMR experiments (Fig. 4B). Thus, the Zn2+·1 chemosensing ensemble serves as a ratiometric sensor for Hg2+ and Pb2+ ions via the competitive metal ion displacement approach. The analytical detection limit11 for Pb2+ under these conditions was 1.6 × 10−8 mol L−1 (Fig. S16 and S17, SI‡). The effect of Zn2+, Hg2+ and Pb2+ with 1 was independently studied with 1H NMR spectroscopy in CD3OD (Fig. 4B). In the presence of Zn2+, small downfield shifts of the ethylenediamine and the coumarin Ha protons were observed indicating their proximity to the binding site. With 1, Hg2+ and Pb2+ induced a significant upfield shift of the methylene protons next to the amide linkage (Hb), and a downfield shift of methylene protons next to the thiocarbamate moiety (Hc) in addition to the downfield shift of Ha. Besides, when 1 eqv. of Hg2+ (or Pb2+) was added to the Zn2+·1 ensemble, the chemical shifts were almost identical to those of the Hg2+·1 (or Pb2+) complex (Fig. 4B). This is an added proof for the higher affinity of 1 towards Hg2+ and Pb2+ compared to Zn2+. Based on the above results the proposed binding mode of 1 with Zn2+, Hg2+ and Pb2+ is shown in Fig. 4A.
![Change in fluorescence spectrum of Zn2+·1 (10 μmol L−1 of 1 mixed with 20 μmol L−1 of Zn2+) in MeOH/DMSO (99 : 1, v/v) upon addition of (A) Hg2+ and (B) Pb2+ in HEPES buffered at pH 7.0. Inset: (A) (i) Ratio of emission intensity [I415/I495] as a function of eqv. of Hg2+ (ii) and Job's plot between 1 and Hg2+. (B) Ratiometric emission intensity [I410/I495] as a function of eqv. of Pb2+.](/image/article/2012/RA/c2ra20822a/c2ra20822a-f3.gif) | ||
Fig. 3 Change in fluorescence spectrum of Zn2+·1 (10 μmol L−1 of 1 mixed with 20 μmol L−1 of Zn2+) in MeOH/DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) upon addition of (A) Hg2+ and (B) Pb2+ in HEPES buffered at pH 7.0. Inset: (A) (i) Ratio of emission intensity [I415/I495] as a function of eqv. of Hg2+ (ii) and Job's plot between 1 and Hg2+. (B) Ratiometric emission intensity [I410/I495] as a function of eqv. of Pb2+. 1, v/v) upon addition of (A) Hg2+ and (B) Pb2+ in HEPES buffered at pH 7.0. Inset: (A) (i) Ratio of emission intensity [I415/I495] as a function of eqv. of Hg2+ (ii) and Job's plot between 1 and Hg2+. (B) Ratiometric emission intensity [I410/I495] as a function of eqv. of Pb2+. | ||
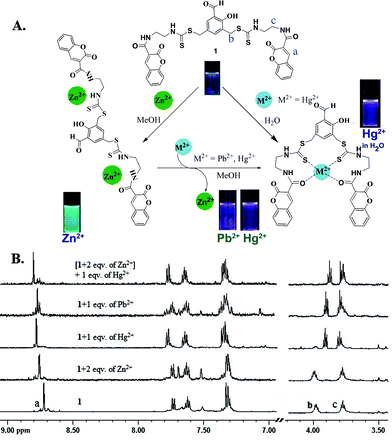 | ||
| Fig. 4 (A) Probable binding model; (B) 1H NMR titration of sensor 1 with Zn2+, Hg2+ and Pb2+. | ||
To determine the stoichiometry, a Job's plot obtained from the emission data showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association of 1 with both Hg2+ and Pb2+ (Inset, Fig. 3(ii) and Fig. S8, SI‡). Furthermore, the result is consistent with the ESI-MS experiment of 1 with excess Pb2+ and Hg2+ (Fig. S21 and S22, SI‡). Under these conditions, the binding constant for Hg2+ and Pb2+ was found to be 3.08 × 105 M−1 and 3.90 × 105 M−1 respectively (Fig. S12 and S13, SI‡).12
1 association of 1 with both Hg2+ and Pb2+ (Inset, Fig. 3(ii) and Fig. S8, SI‡). Furthermore, the result is consistent with the ESI-MS experiment of 1 with excess Pb2+ and Hg2+ (Fig. S21 and S22, SI‡). Under these conditions, the binding constant for Hg2+ and Pb2+ was found to be 3.08 × 105 M−1 and 3.90 × 105 M−1 respectively (Fig. S12 and S13, SI‡).12
The fluorescence behaviour of 1 was then studied in the presence of various metal ions (Na+, K+, Al3+, Ca2+, Cd2+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Li+, Ba2+, Ag+ and Hg2+) in aqueous medium (H2O/DMSO, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 5A). The emission spectrum (λex = 320 nm) of 1 in H2O showed a weak fluorescence band (Φf = 0.028) at 410 nm. Interestingly, among the above metal ions, only the Hg2+ ion enhanced the fluorescence signal remarkably (Φf = 0.48, 17-fold) with a 5 nm red shift in the fluorescence peak (Fig. 5B). The fluorescence intensity increased linearly with the addition up to one equivalent of Hg2+ ions (Fig. 5B, inset). There was no significant change in the fluorescence spectra with any of the other metal ions except for Ag+, which showed a small enhancement in the emission spectra (Fig. 5A).
1, v/v) (Fig. 5A). The emission spectrum (λex = 320 nm) of 1 in H2O showed a weak fluorescence band (Φf = 0.028) at 410 nm. Interestingly, among the above metal ions, only the Hg2+ ion enhanced the fluorescence signal remarkably (Φf = 0.48, 17-fold) with a 5 nm red shift in the fluorescence peak (Fig. 5B). The fluorescence intensity increased linearly with the addition up to one equivalent of Hg2+ ions (Fig. 5B, inset). There was no significant change in the fluorescence spectra with any of the other metal ions except for Ag+, which showed a small enhancement in the emission spectra (Fig. 5A).
 | ||
| Fig. 5 (A) Emission spectra of 1 (10 μM) in aqueous medium upon addition of metal ions (20 μmol L−1). (B) Changes in the emission spectra of 1 (10 μmol L−1) in aqueous medium with Hg2+ (0 to 40 μmol L−1) in the presence of HEPES buffer at pH = 7.0. Inset: Hg2+ titration profile at 415 nm. | ||
Therefore, in aqueous medium, sensor 1 is highly specific towards Hg2+. Under aqueous conditions, Pb2+ does not display any fluorescence enhancement, and thus can clearly be distinguished from Hg2+. Competitive experiments were also carried out to investigate the effects of other coexisting cations along with Hg2+ in the presence of the chemosensor (Fig. S6, SI‡). The results clearly demonstrate that the fluorescence response of 1 is not affected in the presence of other metal ions in aqueous medium. The analytical detection limit for Hg2+ with 1 in aqueous medium was found to have a remarkably low value of 9 nmol L−1 (Fig. S17, SI‡).11 Under aqueous conditions, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association of Hg2+ with 1 was confirmed by a Job's plot (Fig. S9, SI‡) and a mass spectrum (Fig. S22, SI‡). The NMR spectrum of 1 with Hg2+ in D2O/DMSO-d6 showed a larger downfield shift of several protons compared to the ones in CD3OD indicating a stronger association between 1 and Hg2+ in aqueous medium (Fig. S10, SI‡). Indeed, the binding constant in water was found to be 1.2 × 106 M−1 following a Benesi–Hildebrand analysis (Fig. S14, SI‡).12
1 association of Hg2+ with 1 was confirmed by a Job's plot (Fig. S9, SI‡) and a mass spectrum (Fig. S22, SI‡). The NMR spectrum of 1 with Hg2+ in D2O/DMSO-d6 showed a larger downfield shift of several protons compared to the ones in CD3OD indicating a stronger association between 1 and Hg2+ in aqueous medium (Fig. S10, SI‡). Indeed, the binding constant in water was found to be 1.2 × 106 M−1 following a Benesi–Hildebrand analysis (Fig. S14, SI‡).12
The pH dependence of the chemosensor was investigated. Both 1 as well as the Hg2+ complex displayed their fluorescence behaviour from pH 2 to 8 (Fig. S18, SI‡). This indicates that the PET process perhaps involves an electron transfer from the thiocarbamate sulphur to the fluorophore. We envisaged that compound 1 might cross the cell membrane efficiently at physiological pH since the molecule is uncharged and less polar, and after complexation with Hg2+, owing to its charged nature it might stay inside the cell. Thus, 1 was tested for the recognition of Hg2+ ions in cells using fluorescence imaging experiments. HeLa S3 cell lines were exposed to Hg2+ (500 μmol L−1) in DMEM for 2 h at 37 °C (see SI‡). Subsequently, the cells were washed twice to remove the remaining Hg2+ ions, and incubated with 1 (10 μM) in PBS for 10 min at 25 °C. The cells were washed again with PBS to remove any residual dye from the cells. The epifluorescence image of HeLa S3 loaded with 1 (10 μmol L−1) under the same conditions showed negligible intracellular fluorescence (Fig. 6B). In contrast, the cells treated with Hg2+ and 1 revealed highly enhanced intracellular fluorescence (Fig. 6C). These results demonstrate that 1 can be used to detect Hg2+ in living cells.
 | ||
| Fig. 6 Fluorescence images of HeLa S3: (A) only cells, (B) cells with 1 (10 μM), (C) 1- stained cells exposed to Hg2+. | ||
In conclusion, we have synthesized a coumarin based new fluorescence probe 1 that detects multiple metal ions in organic and aqueous media. In methanol sensor 1 shows a ratiometric response to Zn2+. Differentiation of the metal ions under different conditions is summarized in Table 1. Furthermore, Hg2+ and Pb2+ displace Zn2+ from the Zn2+·1 ensemble showing a second ratiometric fluorescence response with isoemission points at 480 and 465 nm. In water, chemosensor 1 exclusively detects Hg2+ demonstrating its excellent selectivity for Hg2+. The sulphur donor in the PET ensures the sensor activity even at low pH. The detection limit of Hg2+ in water of 9 nmol L−1 allows it to detect the minimum level as set by the US-EPA for drinking water. The highly enhanced intracellular fluorescence of 1 in the presence of mercury ions demonstrates that 1 can detect Hg2+ in living cells.
| Metal salt in | Enhancement atb | Inferencec |
|---|---|---|
| a In addition, Zn2+·1 displays ratiometric behaviour with Pb2+/Hg2+. b The values in parentheses are the Φf values; λex = 320 nm, slit 5/5 nm. c The values in parentheses are the Φf values in presence of the respective metal ions. See text. | ||
| 1 in MeOHa | (a) 495 nm (—) | Zn2+ (0.50) |
| (b) 410 nm (0.12) | Pb2+/Hg2+ (0.058/0.039) | |
| 1 in water | (c) 415 nm (0.03) | Hg2+ (0.48) |
| (d) but not (c) | Pb2+ | |
The authors thank DST, India for financial support, IISER for facilities, and CSIR for a SRF to JH.
References
- (a) A. W. Czarnik, Acc. Chem. Res., 1994, 27, 302 CrossRef CAS; (b) B. Wang and E. J. Anslyn, Chemosensors: Principles, Strategies, and Applications, John Wiley and Sons, New York, 2011. Search PubMed.
- (a) X. Liu, M. Zhu, J. Li, X. Yin, H. Zheng, Z. Zuo, C. Ouyang, H. Liu, Y. Li and D. Zhu, J. Org. Chem., 2008, 73, 5008 CrossRef; (b) Y. Chen, L. Wan, X. Yu, W. Li, Y. Bian and J. Jiang, Org. Lett., 2011, 13, 5774 CrossRef CAS; (c) D. Maity and T. Govindaraju, Chem. Commun., 2012, 48, 1039 RSC; (d) L. Xue, Q. Liu and H. Jiang, Org. Lett., 2009, 11, 3454 CrossRef CAS; (e) M.-M. Yu, Z.-X. Li, L.-H. Wei, D.-H. Wei and M.-S. Tang, Org. Lett., 2008, 10, 5115 CrossRef CAS; (f) R. Pandey, R. K. Gupta, M. Shahid, B. Maiti, A. Misra and D. S. Pandey, Inorg. Chem., 2012, 51, 298 CrossRef CAS; (g) P. Das, A. Ghosh, H. Bhatt and A. Das, RSC Adv., 2012, 2, 3714 RSC.
- A. P. de Silva, Chem.–Asian J., 2011, 6, 750 CrossRef.
- B. K. Bitanihirwe and M. G. Cunningham, Synapse, 2009, 63, 1029 CrossRef CAS.
- A. S. Prasad, Br. Med. J., 2003, 326, 409 CrossRef.
- G. J. Fosmire, Am. J. Clin. Nutr., 1990, 51, 225 CAS.
- http://water.epa.gov/drink/contaminants/index.cfm; last accessed 26 Mar 2012.
- Y. Lu, S. Huang, Y. Liu, S. He, L. Zhao and X. Zeng, Org. Lett., 2011, 13, 5274 CrossRef CAS.
- P. Job, Ann. Chim., 1928, 9, 113 CAS.
- (a) S. Lehrer and G. D. Fashman, Biochem. Biophys. Res. Commun., 1966, 23, 133 CrossRef CAS; (b) D. M. Chipman, V. Grisaro and N. Shanon, J. Biol. Chem., 1967, 242, 4388 CAS; (c) S. Saha, A. Ghosh, P. Mahato, S. Mishra, S. K. Mishra, E. Suresh, S. Das and A. Das, Org. Lett., 2010, 12, 3406 CrossRef CAS.
- A. Caballero, R. Martinez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tarranga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666 CrossRef CAS.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS; (b) M. Barra, C. Bohne and J. C. Scaiano, J. Am. Chem. Soc., 1990, 112, 8075 CrossRef CAS.
Footnotes |
| † Dedicated to Professor H. Junjappa. |
| ‡ Electronic supplementary information (ESI) available: details of experimental procedures and characterization data for the products and spectroscopic methods and data. See DOI: 10.1039/c2ra20822a |
| This journal is © The Royal Society of Chemistry 2012 |
