TWEEN coated NaYF4:Yb,Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery†
Wenlu
Ren
a,
Gan
Tian
ac,
Shan
Jian
e,
Zhanjun
Gu
*a,
Liangjun
Zhou
ad,
Liang
Yan
a,
Shan
Jin
ad,
Wenyan
Yin
a and
Yuliang
Zhao
*ab
aKey Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, 100049, P. R. China. E-mail: zjgu@ ihep.ac.cn; zhaoyuliang@ihep.ac.cn
bKey Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanosciences and Technology of China, Beijing, 100190, China
cCollege of Chemistry, Sichuan University, Chengdu, 610064, P. R. China
dCollege of Materials Science and Opto-Electronic Technology, Graduate University of Chinese Academy of Sciences, Beijing, 100049, P. R. China
ePeking Union Medical College Hospital, Beijing, 100730, P. R. China
First published on 12th July 2012
Abstract
By using a commercially available surfactant (TWEEN), we report a simple and efficient method to convert hydrophobic NaYF4:Yb,Er/NaYF4 core/shell upconversion nanoparticles to hydrophilic ones for bioimaging and drug delivery.
Recently, near-infrared (NIR)-to-visible upconversion nanoparticles (UCNPs) have attracted significant interest because of their many unique advantages as bioprobes, such as being free of background autofluorescence, deep light penetration depth, minimal photodamage to living organisms, superior photostability, large anti-Stokes shifts, good chemical/physical stability, and low toxicity.1 Consequently, UCNPs are becoming a new class of powerful tools in biological and medical applications, mainly including cellular labeling,2,3in vivo imaging,4,5 FRET-based biosensors,6,7 drug delivery1b,8 and photodynamic therapy.9 These applications, however, require UCNPs that are bio-compatible, water-soluble and have intense upconversion luminescence. Unfortunately, those high-quality UCNPs with controlled size and excellent luminescence properties are often synthesized in an organic phase and stabilized with hydrophobic ligands, such as oleic acid (OA), thus creating a need to further engineer nanocrystal coatings. In the past few years, several approaches have been developed to modify the surface of hydrophobic UCNPs to make them hydrophilic. Silica coatings and amphiphilic poly (acrylic acid) (PAA) encapsulation have successfully resulted in water dispersibility of UCNPs but these methods failed to prevent aggregation in the presence of serum.10 Poly (ethylene imine) (PEI) was also utilized as a stabilizer for the UCNPs, but the cytotoxicity of PEI greatly limits its application for in vivo studies.11 UCNPs coated with PEGylated amphiphilic polymers have been proven to be bio-compatible and have very high stability in aqueous solution and other biological fluids.8,12 However, currently used PEGylated polymers for UCNP coatings require custom synthesis or are difficult to obtain in biological laboratories.
Here, we report a facile and cost effective surface modification approach that uses a commercially available surfactant (TWEEN) as the stabilizer to produce water-soluble and bio-compatible UCNPs. These TWEEN compounds are commonly used in the food industry and biochemical analysis due to their relative nontoxicity and low price.13 But no one has, to our knowledge, evaluated their abilities to keep UCNPs well dispersed in biological solutions. TWEEN compounds are composed of three chemical parts: aliphatic ester chains that can prevent nonspecific adsorption of protein, three-terminal hydroxyl groups that are hydrophilic and can be chemically modified for further applications, as well as an aliphatic chain that can easily be adsorbed on the hydrophobic surface of UCNPs by hydrophobic interactions (Scheme 1a and 1c). This structure makes TWEEN unique for coating UCNPs to render them water soluble for use in biomedical applications. Our results clearly show that the TWEEN coated NaYF4:Yb,Er/NaYF4 core/shell UCNPs exhibit good water-dispersibility and bio-compatibility. In addition, TWEEN coated UCNPs do not show notable reduction in the overall luminescence intensity under excitation at 980 nm compared to the original OA-capped UCNPs. This will facilitate their application in bioimaging and bio-analysis. Furthermore, these TWEEN-UCNPs could also be employed as drug delivery carriers. A commonly used chemotherapeutic drug, doxorubicin (DOX), was loaded on these TWEEN-UCNPs by physical adsorption through hydrophobic interaction (Scheme 1b). Thus, TWEEN coated UCNPs could be employed as a promising multifunctional nanoplatform for simultaneous diagnosis and therapy.
 | ||
| Scheme 1 (a) Formation of water-soluble UCNPs by coating with TWEEN 80. (b) DOX loaded TWEEN 80/UCNPs. The hydrophobic layer on the nanoparticle surface allows adsorption of drug molecules on the UCNPs through hydrophobic interactions. (c) Chemical structures of TWEEN 80 and DOX. | ||
The NaYF4:Yb/Er UCNPs were synthesized using a modified method reported by Jalil and Zhang.14 The morphology and size of the nanoparticles are shown in Fig. 1a, which clearly reveals that these nanocrystals are spherical, highly monodisperse, and narrow in size distribution. The average diameter of the nanoparticles was estimated to be 8 nm. Unfortunately, these small UCNPs showed relative weak upconversion emission that is easily quenched in aqueous solution. To improve the intensity and reduce the quenching of the upconversion emission, an undoped shell of NaYF4 was grown over the core NaYF4:Yb/Er nanoparticles. This shell can protect the luminescent ions in the core (especially those near the surface) from non-radiative decay caused by surface defects as well as from vibrational deactivation from solvents in the colloidal dispersions. The average diameters of the core/shell nanocrystals increased to 25 nm while still keeping their uniform shape and narrow size distribution (Fig. 1b). The X-ray diffraction pattern as shown in Fig. 1c indicates that all of the diffraction peak of the core/shell nanoparticles can be indexed to the pure hexagonal phase of NaYF4 (JCPDS card no. 16-0334), which is regarded as the most effective host matrix for upconversion fluorescence due to its low phonon energy. Fig. 1d shows the corresponding upconversion emission spectra of the core-only and core/shell nanoparticles. In both cases, two distinct Er3+ emission bands are observed. The green emissions in the region of 520–541 nm and 545–561 nm are attributed to the transitions 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 of Er3+ ions, respectively, whereas the red emission between 645–683 nm corresponds to the 4F9/2 → 4I15/2 transition.15,16 Notably, the intensity of upconversion emission from the core/shell nanocrystals is as much as 16 times brighter than that of the core-only nanoparticles, which may greatly favor their application in bioimaging and bio-analysis.
 | ||
| Fig. 1 TEM images of (a) core: NaYF4: 20%Yb, 2%Er UCNPs (b) core/shell NaYF4: 20%Yb, 2%Er/NaYF4 nanocrystals. (c) XRD pattern of core/shell nanocrystals. (d) Upconversion luminescence spectra of core and core/shell nanoparticles upon 980 nm excitation. All samples (dispersed in cyclohexane) were of the same wt% for comparison. | ||
The surfactant TWEEN 80 was chosen to transfer the hydrophobic UCNPs into the aqueous phase because of its low price, bio-compatibility and the ability to prevent the non-specific adsorption of proteins due to its unique molecular structure. The coating of TWEEN on the UCNPs was formed by hydrophobic interactions between the fatty-acid tails of TWEEN and the oleic acid layer on the nanoparticle surface. The successful coating was evidenced by IR spectra (see Fig. S1†). After coating, these UCNPs possessed good dispersibility in water, as shown in Fig. 2a and 2b. We also examined if TWEEN coated UCNPs can stably disperse in other bio-relevant fluids that contain proteins. It is well known that, if without any protection, many type of proteins tend to non-specifically adsorb on the nanoparticle surface, which is one of the biggest problems in nanoparticle-based biological applications including biosensors, drug delivery and biomedicine. In our case, as shown in Fig. 2c, TWEEN modified UCNPs dispersed well in bio-buffers (phosphate buffer solution and fetal bovine serum) and in cell culture media (Dulbecco modified eagle medium) and no observable aggregation of nanoparticles was found after 48 h. These results revealed that TWEEN could protect the nanoparticles from non-specific adsorption of proteins and provide them with good dispersibility in various biological solutions. In addition, the TWEEN modified UCNPs dispersed in water retained almost an equivalent upconversion emission intensity to that of the OA-capped UCNPs in cyclohexane (Fig. 2d). The reason for decreased quenching of upconversion emission in water after TWEEN coating may be that the hydrophobic layer (composed of oleic acid and the fatty-acid tails of TWEEN) on the surface of the UCNPs restricted the access of water molecules to the emitting lanthanide ions resulting in significantly reduced quenching when compared with UCNPs modified by ligand exchange.17Fig. 2e shows a TEM image of the TWEEN-UCNPs, which indicates the surface modification has no obvious effect on their size and shape.
 | ||
| Fig. 2 Photographs of (a) TWEEN-UCNPs dispersed in water. (b) Upconversion luminescence of TWEEN-UCNPs in water excited with a 980 nm laser. (c) TWEEN-UCNPs dispersed in buffers (PBS and FBS) and cell culture media (DMEM). (d) Upconversion luminescence spectra of OA capped UCNPs dispersed in cyclohexane and TWEEN coated UCNPs dispersed in water, respectively. Both samples are excited under the same conditions (980 nm excitation source). (e) TEM image of TWEEN coated UCNPs. | ||
Next, we evaluated the cytotoxicity characteristics of TWEEN-UCNPs using a standard Cell Counting Kit-8 (CCK-8) colorimetric assay with A549 cells, which is an important issue towards the biological applications of such materials as bioprobes. After 24 h incubation with the nanoparticle concentration varying from 6.25 to 200 μg mL−1, the TWEEN-UCNPs exhibited satisfactory biocompatibility as shown in Fig. 3a. The viability of A549 cells remained above 80%, whatever the dose used, even at a concentration of 200 μg mL−1. These results clearly reveal that TWEEN coated NaYF4 UCNPs have low cytotoxicity, which is consistent with previous reports.13,14 By using a modified inverted microscope equipped with a 980 nm NIR laser, the further practical application of TWEEN-UCNPs in cell imaging was investigated. After incubation with 100 μg mL−1 TWEEN-UCNPs for 2 h, NIR-to-visible upconversion fluorescence was clearly observed in the cells with high signal-to-background ratio because biological samples have very low absorption of 980 nm light (Fig. 3b). The overlays of bright-field and upconversion luminescent images further indicated that the luminescence came from the intracellular region, suggesting the TWEEN-UCNPs were internalized into the cells. All these results suggest these nanoparticles are promising probes for cell imaging due to their low toxicity and high signal-to-noise ratio under 980 nm NIR excitation.
 | ||
| Fig. 3 (a) In vitro cell viability of A549 cells incubated with TWEEN-UCNPs at different concentrations for 24 h. (b) Inverted fluorescence microscope images of HeLa cells labeled with TWEEN coated UCNPs under 980 nm laser excitation. | ||
TWEEN-UCNPs prepared by our method have a hydrophobic layer on the surface of the nanoparticles. Thus, these nanoparticles could be employed as drug delivery carriers since lipophilic molecules, such as doxorubicin (DOX), could be absorbed into this layer through hydrophobic interactions.1b,8 The nanoparticles were soaked in a concentrated solution of DOX for 24 h and the loading efficiency of adsorption of DOX onto TWEEN-UCNPs in phosphate buffer (PBS, pH 7.4) was about 7.4 wt% (Fig. 4a), which was determined by measuring the characteristic absorption peak of DOX at 480 nm (Fig. 4b). The color of the nanoparticles turned from white to orange after DOX loading, which further confirmed the successful adsorption of DOX onto the nanoparticles. The in vitro release of DOX from the DOX-loaded TWEEN-UCNPs complex was examined in PBS at different pH values as illustrated in Fig. 4c. In neutral PBS (pH 7.4), to simulate normal physiological conditions, DOX adsorbed on TWEEN-UCNPs was released very slowly and the amount of released DOX, presented as a weight percentage of the total DOX adsorbed, was only about 40% over a period of 72 h. In contrast, in PBS at pH 5.0, to simulate the intracellular conditions of cancer cells, the release rate of DOX from TWEEN-UCNPs became much faster and the highest release of DOX was over 70% within 72 h. These results suggest that the release of DOX from the nanoparticles could be controlled by varying the pH, which may facilitate its drug delivery and controlled release into cancer cells since the microenvironments in the extracellular tissues of tumors and intracellular lysosomes and endosomes are acidic. Next, we testify the cancer killing ability of the DOX@TWEEN-UCNPs complex. Fig. 4d shows the viability of A549 cells exposed to the free DOX and DOX@TWEEN-UCNPs at various concentrations after incubation time of 12 h, respectively. The dose-dependent cell viability was observed in both the DOX-only group and DOX@TWEEN-UCNPs group. When compared with free DOX, DOX@TWEEN-UCNPs exhibit better efficacy to cause cancer death. The enhanced efficacy may be due to the delivery of more DOX into the cell by the nanoparticle-based platform than when using DOX alone. Similar phenomena have been encountered in many previous reports.18 Thus, we conclude that the TWEEN-UCNPs are a promising drug delivery carrier for cancer therapy.
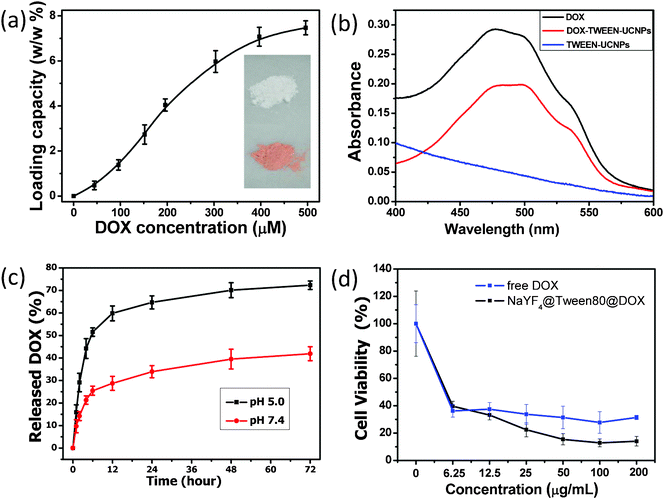 | ||
| Fig. 4 Drug loading and release behavior study. (a) Quantification of DOX loading at different DOX concentrations in PBS (pH 7.4). TWEEN-UCNPs dispersions of the same concentrations (5 mg mL−1) were used in this experiment. (b) UV-Vis absorbance spectra of free DOX, bare TWEEN-UCNPs and DOX@TWEEN-UCNPs. (c) Real time monitoring of DOX release from DOX@TWEEN-UCNPs in PBS at two different pH values. Acidic medium significantly accelerated the DOX release rate. Error bars are based on standard deviations of triplicated samples. (d) Concentration-dependent cell viability of A-549 cells treated with free DOX and DOX@TWEEN-UCNPs. Inset in Fig. 4a shows the photographs of TWEEN-UCNPs before and after loading with DOX. | ||
In summary, we have successfully developed a simple yet effective method to convert hydrophobic UCNPs into hydrophilic ones using a commercially available surfactant (TWEEN). The presence of adsorbed TWEEN can greatly improve the dispersibility of UCNPs in water, biological buffers and cell culture media. Notably, the upconversion efficiency of TWEEN coated NaYF4:Yb,Er/NaYF4 core/shell nanocrystals was almost unchanged in aqueous solution compared with hydrophobic ones in the organic environment. So, the TWEEN-UCNPs exhibit good capability for bioimaging due to their good water-dispersibility and intense upconversion emissions. In addition, these TWEEN coated UCNPs could also be employed as drug delivery carriers. DOX, a lipophilic anticancer drug, could be adsorbed on these TWEEN-UCNPs through hydrophobic interactions. The release of DOX from UCNPs can be regulated by varying the pH. As a result, the as-prepared TWEEN coated UCNPs bearing uniform shape, narrow size distribution, efficient upconversion luminescence, good water solubility, low toxicity and drug delivery capability, could be potentially employed as a multifunctional platform for simultaneous diagnosis and therapeutic application.
This work was supported by National Basic Research Programs of China (973 program, No. 2012CB932504, 2011CB933403 and 2012CB934001), and National Natural Science Foundation of China (No. 21001108, 21177128 and 21101158).
References
- (a) Q. Liu, C. Y. Li, T. S. Yang, T. Yi and F. Y. Li, Chem. Commun., 2010, 46, 5551–5553 RSC; (b) G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS; (c) Z. Q. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606–345610 CrossRef; (d) F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS.
- Q. T. Chen, X. Wang, F. H. Chen, Q. B. Zhang, B. Dong, H. Yang, G. X. Liu and Y. M. Zhu, J. Mater. Chem., 2011, 21, 7661–7667 RSC.
- T. Y. Cao, Y. Yang, Y. Gao, J. Zhou, Z. Q. Li and F. Y. Li, Biomaterials, 2011, 32, 2959–2968 CrossRef CAS.
- J. C. Zhou, Z. L. Yang, W. Dong, R. J. Tang, L. D. Sun and C. H. Yan, Biomaterials, 2011, 32, 9059–9067 CrossRef CAS.
- G. Zhang, Y. L. Liu, Q. H. Yuan, C. H. Zong, J. H. Liu and L. H. Lu, Nanoscale, 2011, 3, 4365–4371 RSC.
- L. Cheng, K. Yang, M. W. Shao, S.-T. Lee and Z. Liu, J. Phys. Chem. C, 2011, 115, 2686–2692 CAS.
- S. Jiang and Y. Zhang, Langmuir, 2010, 26, 6689–6694 CrossRef CAS.
- C. Wang, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 1110–1120 CrossRef CAS.
- M. E. Lim, Y.-L. Lee, Y. Zhang and J. J. H. Chu, Biomaterials, 2012, 33, 1912–1920 CrossRef CAS.
- J. Shan, J. Chen, J. Meng, J. Collins, W. Soboyejo, J. S. Friedberg and Y. Ju, J. Appl. Phys., 2008, 104, 094308 CrossRef.
- C. Brunot, L. Ponsonnet, C. Lagneau, P. Farge, C. Picart and B. Grosgogeat, Biomaterials, 2007, 28, 632–640 CrossRef CAS.
- S. J. Budijono, J. N. Shan, N. Yao, Y. Miura, T. Hoye, R. H. Austin, Y. G. Ju and R. K. Prud'homme, Chem. Mater., 2010, 22, 311–318 CrossRef CAS.
- (a) S. Park, N. Mohanty, J. W. Suk, A. Nagaraja, J. H. An, R. D. Piner, W. W. Cai, D. R. Dreyer, V. Berry and R. S. Ruoff, Adv. Mater., 2010, 22, 1736–1740 CrossRef CAS; (b) W. N. Burnette, Anal. Biochem., 1981, 112, 195–203 CrossRef CAS; (c) E. Engvall and P. Perlman, Immunochemistry, 1971, 8, 871–874 CrossRef CAS.
- R. A. Jalil and Y. Zhang, Biomaterials, 2008, 29, 4122–4128 CrossRef.
- Z. Q. Li and Y. Zhang, Angew. Chem., Int. Ed., 2006, 45, 7732–7735 CrossRef CAS.
- C. R-Lecuna, R. M-Rodríguez and R. Valiente, Chem. Mater., 2011, 23, 3442–3448 CrossRef.
- (a) G. S. Yi and G. M. Chow, Chem. Mater., 2007, 19, 341–343 CrossRef CAS; (b) G. C. Jiang, J. Pichaandi, N. J. J. Johnson, R. D. Burke and F. C. J. M. V. Veggel, Langmuir, 2012, 28, 3239–3247 CrossRef CAS.
- (a) Q. J. He, Y. Gao, L. X. Zhang, Z. W. Zhang, F. Gao, X. F. Ji, Y. P. Li and J. L. Shi, Biomaterials, 2011, 32, 7711–7720 CrossRef CAS; (b) J. N. Shen, Q. J. He, Y. Gao, J. L. Shi and Y. P. Li, Nanoscale, 2011, 3, 4314–4322 RSC; (c) W. H. Di, X. G. Ren, H. F. Zhao, N. Shirahata, Y. Sakka and W. P. Qin, Biomaterials, 2011, 32, 7226–7233 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; FTIR spectra of TWEEN 80 coated UCNPs and pure TWEEN 80 (Fig. S1); chemical structure of TWEEN series compounds (Fig. S2); photographs of phase transfer of UCNPs from cyclohexane to water upon addition of TWEEN 80 (Fig. S3). See DOI: 10.1039/c2ra20855e |
| This journal is © The Royal Society of Chemistry 2012 |
