A novel fluorescent probe with high sensitivity and selective detection of lipid hydroperoxides in cells
Kazunori
Yamanaka
a,
Yoshiro
Saito
a,
Junji
Sakiyama
b,
Yuya
Ohuchi
b,
Fumio
Oseto
b and
Noriko
Noguchi
*a
aSystems Life Sciences, Department of Medical Life Systems, Faculty of Medical and Life Sciences, Doshisha University, Kyotanabe 610-0394, Japan. E-mail: nnoguchi@mail.doshisha.ac.jp; Fax: +81-774-65-6262
bDojindo Laboratories, Kumamoto, Japan
First published on 1st August 2012
Abstract
Spy-LHP was developed as a fluorescent probe for the detection and imaging of lipid hydroperoxides in living cells. Although Spy-LHP detection of lipid hydroperoxides is sensitive and selective, this probe is unsuitable for live-cell imaging because of its high hydrophobicity. To overcome this limitation, 2-(4-diphenylphosphanyl-phenyl)-9-(3,6,9,12-tetraoxatridecyl)-anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10-tetraone, Liperfluo, was developed. Liperfluo is structurally similar to Spy-LHP, but is much more soluble in various organic solvents, such as ethanol and dimethylsulfoxide. The probe was successfully used to image lipid hydroperoxides in SH-SY5Y cells in which lipid peroxidation had been stimulated with 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride or cumene hydroperoxide by using fluorescent microscopy and confocal laser scanning microscopy. Additionally, flow cytometric analysis showed that Liperfluo was four times more sensitive in detecting lipid hydroperoxides than Spy-LHP. These results suggest that Liperfluo is a useful fluorescent probe for investigating the roles of lipid peroxidation in a variety of cell pathophysiologies.
Introduction
Lipid peroxidation induced by reactive oxygen species (ROS) and/or by reactive nitrogen species (RNS) under oxidative stress is a causative factor of aging and various diseases including cancer, atherosclerosis, and neurodegenerative diseases.1,2 Lipid hydroperoxides are the primary products of lipid peroxidation and are important markers of oxidative stress. Several methods for detecting lipid hydroperoxides using cyclooxygenase,3,4 glutathione peroxidase,5 and high-performance liquid chromatography (HPLC) with detection by ultraviolet light and chemiluminesence6 have been developed. However, detection of lipid hydroperoxides in living cells remains to be improved.Fluorescent probes are promising tools for detecting oxidative stress in cells because of their sensitivity and selectivity. Several types of fluorescent probe for detecting ROS and RNS have been developed. 2′,7′-Dichlorodihydrofluorescin diacetate (DCFH-DA) is a well-known fluorescent probe used for evaluating oxidative stress in cells.7 DCFH-DA is incorporated into cells and hydrolyzed by esterase, resulting in the generation of DCFH. DCFH is oxidized by reacting with hydroxyl radicals, alkoxyl radicals, and peroxyl radicals to form fluorescent DCF; however, this oxidation reaction does not occur with hydrogen peroxide and lipid hydroperoxides.8 Since DCFH is located in both the cytosol and membranes, it reacts with both hydrophilic and hydrophobic reactive species. Therefore, DCFH is suitable for detecting total free radicals regardless of the cellular compartment being examined.
Diphenyl-1-pyrenylphosphine (DPPP)9 is one of the most widely used probes for detecting lipid peroxidation in various biosamples.10–13 However, DPPP has a short excitation wavelength (∼352 nm), which causes damage to living cells. Additionally, cell imaging using this probe requires a specific type of CCD camera. To overcome these undesirable properties, 2-(4-diphenylphosphanyl-phenyl)-9-(1-hexyl-heptyl)-anthra [2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10-tetraone (Spy-LHP; named after the “swallow-tailed perylene derivative for detecting lipid hydroperoxides”) was developed14 (Scheme 1). Spy-LHP selectively and quantitatively reacts with lipid hydroperoxides to form fluorescent Spy-LHPOx, which can be detected at long wavelengths (λex = 524 nm, λem = 535 nm) (Scheme 1). Additionally, this probe can be used for the confocal laser scanning microscopy imaging of lipid hydroperoxides in living J774A.1 cells. However, Spy-LHP is dissolved in highly cytotoxic organic solvents such as acetone and chloroform (CHCl3), but is dissolved poorly in low cytotoxic organic solvents such as ethanol and dimethylsulfoxide (DMSO), which restricts the use of this probe in several cell types. Therefore, the solubility of Spy-LHP must be improved for detection of lipid hydroperoxides in living cells. To overcome these limitations, the novel fluorescent probe Liperfluo was successfully developed (Scheme 1). Here, we report the ideal use of Liperfluo for imaging lipid hydroperoxides in living cells supported by its high solubility in DMSO.
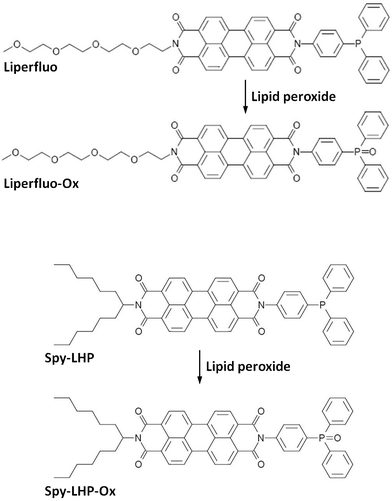 | ||
| Scheme 1 Reaction of Liperfluo and Spy-LHP with lipid hydroperoxides. | ||
Results and discussion
Fluorescent properties of Liperfluo and Liperfluo-Ox
To increase the solubility of Spy-LHP in DMSO, its swallow-tailed moiety was replaced by a tetraethylene glycol group; this compound was referred to as Liperfluo (Scheme 1). We compared the solubility of Liperfluo and Spy-LHP in several solvents, such as CHCl3, ethanol, DMSO, and H2O (Table 1). Liperfluo showed 30-fold higher solubility than Spy-LHP in DMSO, suggesting that Liperfluo is promising for use in living cells.| CHCl3 | Ethanol | DMSO | H2O | |
|---|---|---|---|---|
| Liperfluo | >3 mg ml−1 | <0.03 mg ml−1 | <1.2 mg ml−1 | Insoluble |
| Spy-LHP | >3 mg ml−1 | Slightly soluble | <0.04 mg ml−1 | Insoluble |
The detection of lipid hydroperoxides using Liperfluo is based on an oxidation reaction between Liperfluo and lipid hydroperoxides, resulting in the formation of highly fluorescent Liperfluo-Ox, which is similar to Spy-LHP (Scheme 1). We analyzed the fluorescent properties of Liperfluo; the emission and excitation spectra of Liperfluo and Liperfluo-Ox are shown in Fig. 1. Peak wavelengths for Liperfluo and Liperfluo-Ox are summarized along with fluorescence quantum yields in Table 2. Liperfluo is weakly fluorescent, whereas the fluorescence of Liperfluo-Ox is very strong. Because of the long-range excitation and emission wavelengths of both Liperfluo and Liperfluo-Ox (λex = 524 nm, λem = 535 nm), damage to living cells due to light irradiation and autofluorescence of biomatrices can be avoided.
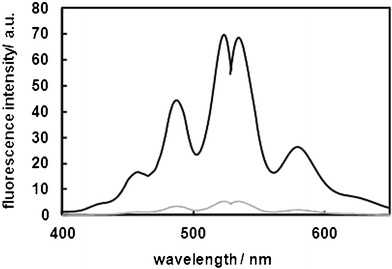 | ||
| Fig. 1 Excitation and emission spectra of Liperfluo and Liperfluo-Ox. Measurements were carried out at room temperature in ethanol. | ||
| A/nm | E m/nm | φ f | |
|---|---|---|---|
| Liperfluo | 524![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487 |
535![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 578 |
0.03 |
| Liperfluo-Ox | 524![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487 |
535![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 579 |
0.85 |
To evaluate the ability of Liperfluo to detect lipid hydroperoxides, 1 μM Liperfluo was reacted with various concentrations of methyl linoleate hydroperoxide (MeLOOH). MeLOOH is a typical lipid hydroperoxide. Fig. 2A shows the fluorescence spectra of Liperfluo (Liperfluo-Ox) after 10 min of incubation with various concentrations of MeLOOH at room temperature. The fluorescence intensity of Liperfluo (Liperfluo-Ox) increased with MeLOOH concentrations in a concentration-dependent manner. A good correlation between the concentration of MeLOOH and fluorescence intensity was obtained (Fig. 2B), which agrees with previous studies examining the fluorescence of Spy-LHP.14
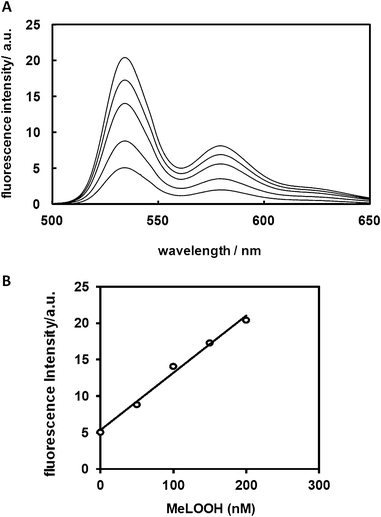 | ||
| Fig. 2 Relationship between fluorescence intensity and concentration of MeLOOH. (A) Emission spectra for Liperfluo at room temperature in ethanol in the presence of MeLOOH at various concentrations. Emission spectra were obtained 10 min after adding MeLOOH to Liperfluo (1 μM) under aerobic conditions at an excitation wavelength of 488 nm. (B) Relationship between fluorescence intensity and concentration of MeLOOH. The fluorescence intensity was measured at 535 nm before and after addition of MeLOOH. | ||
To evaluate the selectivity of Liperfluo for lipid hydroperoxides, Liperfluo was treated with various reactive oxygen species (ROS), such as H2O2, hydroxyl radical (•OH), superoxide anion (O2•−), nitric oxide (NO), peroxynitrite (ONOO−), alkylperoxyl radical (ROO•), cumene hydroperoxide (Cumene-OOH), and MeLOOH. A strong increase in fluorescence intensity was observed only for the reaction of Liperfluo with MeLOOH (Fig. 3). These results indicate that Liperfluo selectively reacts with lipid hydroperoxides.
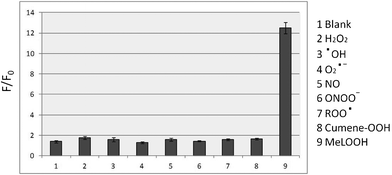 | ||
| Fig. 3 Relative fluorescence intensity (F/F0) of Liperfluo with various ROS in ethanol. Liperfluo (1 μM) was reacted with various ROS (5 μM) for 30 min at room temperature. F0 and F denote fluorescence intensity at 535 nm before and after the addition of various ROS, respectively. | ||
Cytotoxicity of Liperfluo
The cytotoxicity of Liperfluo was assessed by two assay methods, MTT assay and LDH activity assay. SH-SY5Y cells were incubated in culture medium containing up to 80 μM Liperfluo added as a DMSO solution for 15 min. As shown in Fig. 4, Liperfluo possessed little cytotoxicity in SH-SY5Y cells.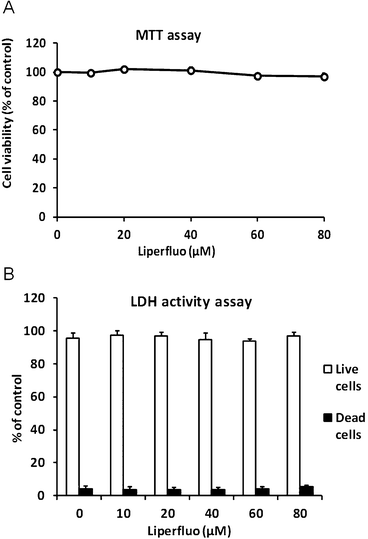 | ||
| Fig. 4 Evaluation of Liperfluo cytotoxicity in SH-SY5Y cells. SH-SY5Y cells were treated with variable concentrations of Liperfluo at 37 °C for 15 min. The medium was replaced by serum-containing medium and incubated at 37 °C for 24 h. The cell viability was measured by (A) MTT assay and (B) LDH activity assay, as described in the Experimental section. | ||
Comparison of Spy-LHP and Liperfluo for cell imaging
The ability of Spy-LHP and Liperfluo to detect lipid hydroperoxides in living cells was examined. CHCl3 and DMSO were selected as suitable solvents for dissolving Spy-LHP and Liperfluo, respectively. Both Spy-LHP and Liperfluo were completely dissolved in CHCl3 up to 10 mM. In contrast, Liperfluo was completely dissolved in DMSO at 1 mM, whereas Spy-LHP was not. The insoluble Spy-LHP in DMSO was removed by centrifugation and the soluble fraction was used for subsequent experiments.SH-SY5Y cells were pre-treated with either Spy-LHP or Liperfluo at a final concentration of 20 μM for 15 min in serum-free medium. DMSO solutions containing either Spy-LHP or Liperfluo were fully miscible in serum-free medium. As indicated by a white arrow in the bright-field image in the optical micrography, SH-SY5Y cells treated with Liperfluo dissolved in DMSO alone look healthy (Fig. 5). However, since CHCl3 solutions of both probes were immiscible in the medium, they were used for SH-SY5Y cells as a suspended form. Cells were not damaged by treatment with any probe added as either a CHCl3 solution or a DMSO solution in the absence of oxidation initiators (data not shown). After the medium containing either probe was removed from the cell samples, the cells were treated with either 6 mM 2,2′-azobis-[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH) or 100 μM Cumene-OOH for 2 h in serum-containing medium. The generation of lipid hydroperoxides in AIPH- and Cumene-OOH-treated cells has been reported.15,16 The cells incubated with either AIPH or Cumene-OOH looked extremely damaged, as indicated by a black arrow in the bright-field image of optical microscopy (Fig. 5). Changes in fluorescence were monitored using fluorescence microscopy (Fig. 5). Cells that had been pre-treated with either Spy-LHP or Liperfluo dissolved in CHCl3 showed minimal fluorescence following AIPH stimulation and a mild increase in fluorescence intensity following Cumene-OOH stimulation. This may be because the probe was heterogeneously distributed in the media due to the high hydrophobicity of CHCl3, thereby preventing the probes from accessing the cell surface. Similarly, cells treated with Spy-LHP dissolved in DMSO showed minimal fluorescence even after stimulation with AIPH or Cumene-OOH. Under all these conditions, cells were damaged (data not shown). It is thought that only a small amount of Spy-LHP was incorporated into the cells due to the poor solubility of Spy-LHP in DMSO. The amount of Spy-LHP dissolved in DMSO was not sufficient for incorporation into the cells or detection of lipid hydroperoxides. In contrast, cells pre-treated with Liperfluo dissolved in DMSO exhibited a distinct increase in fluorescence following stimulation with AIPH or Cumene-OOH. The imaging of lipid hydroperoxides in living cell was obtained using confocal laser scanning microscopy (Fig. 6A). The cell imaging data indicate that the probe is targeting the cell membrane, not the nucleus. Furthermore, we confirmed the different localization of Liperfluo-Ox and DAPI, a nucleus-specific probe, by using fluorescent microscopy. The data are shown in Fig. 6B. These experimental results demonstrate that Liperfluo is more useful as a probe than Spy-LHP for live-cell fluorescence imaging.
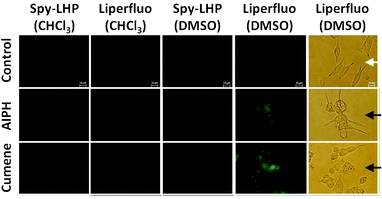 | ||
| Fig. 5 Bright-field and fluorescence images of living SH-SY5Y cells. SH-SY5Y cells were pre-incubated in serum-free medium containing 20 μM Spy-LHP or Liperfluo added to CHCl3 or DMSO at 37 °C for 15 min. The medium was replaced by medium containing serum and either 6 mM AIPH or 100 μM Cumene-OOH and incubated at 37 °C for 2 h. White and black arrows in the bright-field optical microscopy images indicate healthy cells and damaged cells, respectively. The fluorescence images of lipid hydroperoxides generated in live cells were obtained using fluorescence microscopy. | ||
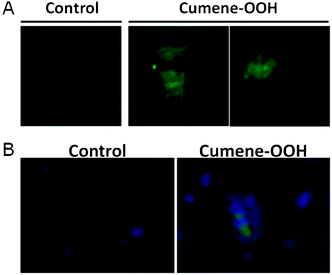 | ||
| Fig. 6 Fluorescence image of lipid hydroperoxides in SH-SY5Y cells treated with Cumene-OOH. SH-SY5Y cells were pre-incubated in serum-free medium containing 20 μM Liperfluo added as a DMSO solution at 37 °C for 15 min. The medium was replaced by medium containing serum and 100 μM Cumene-OOH, and cells were incubated at 37 °C for 2 h. (A) Fluorescence images in living cells were obtained using confocal laser scanning microscopy. (B) Nucleus was stained with DAPI. Fluorescence images in SH-SY5Y cells were obtained using fluorescence microscopy. | ||
Quantitative analysis of lipid hydroperoxides in living cells
For quantitative analysis of lipid hydroperoxide levels in living cells, we performed flow cytometric analysis (Fig. 7). The experimental design was the same as that shown in Fig. 4. SH-SY5Y cells were pre-treated with either Spy-LHP or Liperfluo at a final concentration of 20 μM for 15 min in serum-free medium. After pre-treatment, the cells were treated with 6 mM AIPH or 100 μM Cumene-OOH for 2 h in medium containing serum. When cells pre-treated with either Spy-LHP or Liperfluo in CHCl3 were stimulated with AIPH or Cumene-OOH, fluorescence intensity increased by 1.3- and 2-fold, respectively (Fig. 7A, B, and E). When cells were pre-treated with Spy-LHP in DMSO and then stimulated with either AIPH or Cumene-OOH, we observed a 1.3- or 2-fold increase in fluorescence intensity, respectively (Fig. 7C and E). In contrast, cells pre-treated with Liperfluo in DMSO showed an increased fluorescence intensity of approximately 1.7-fold and 4.7-fold following stimulation with AIPH or Cumene-OOH, respectively (Fig. 7D and E). This observation agrees with those of fluorescent imaging using a microscope.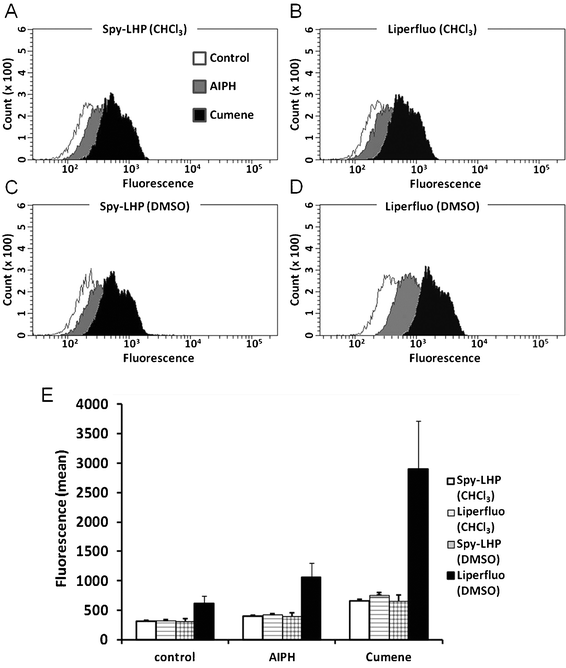 | ||
| Fig. 7 Flow cytometric analysis of lipid hydroperoxides in live cells. SH-SY5Y cells were treated as described in Fig. 5. Fluorescence measurements were performed using a BD FACSAriaTM II cell sorter. Representative data from at least three independent experiments are shown (A–D). The fluorescent isothiocyanate (FITC) means in these cells measured using flow cytometry are shown. Each value represents the mean ±SD of three independent experiments (E). | ||
As shown in Fig. 3, the selectivity of Liperfluo for lipid hydroperoxides was observed. The generation of oxidation products of linoleate and cholesterol in AIPH-treated cells has been reported;15 therefore, it is considered that hydroperoxides of these lipids are candidates to react with Liperfluo. Lipid peroxidation in Cumene-OOH-treated cells has also been reported.16 Since Liperfluo reacted with lipid hydroperoxides but not Cumene-OOH (Fig. 3), it is thought that Liperfluo reacts with lipid hydroperoxides in cells treated with Cumene-OOH.
Lipid hydroperoxides, which are primary products of lipid peroxidation and sources of highly toxic secondary products such as aldehydes, play important roles in the pathophysiology of cells.17 In the presence of transition metals, they are decomposed to generate lipid alkoxyl radicals and peroxyl radicals.17 They also activate lipoxygenases to induce lipid peroxidation,18 while lipid hydroperoxides are the substrates for many enzymes such as glutathione peroxidase.19 Liperfluo reduces lipid hydroperoxides to lipid alcohols, which are relatively stable and minimally toxic. Similarly to DPPP, Liperfluo may be used to reduce lipid hydroperoxides to examine their roles in gene expression regulation, as reported in a previous paper;20 additionally, Liperfluo is less cytotoxic than DPPP.
Conclusion
We report a novel fluorescent probe, Liperfluo, which is extremely useful for imaging lipid hydroperoxides in living cells because of its high solubility in hydrophilic organic solvents. Liperfluo reduces lipid hydroperoxides to lipid alcohols and can be a powerful tool to investigate the roles of lipid peroxidation in a variety of pathophysiologies of cells.Experimental
Reagents
Potassium superoxide was from Strem Chemicals (Newburyport, MA). Hydrogen peroxide, potassium superoxide, 2,2-azobis(2-amidinopropane)dihydrochloride (AAPH), 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH), and methyl linoleate were from Wako Pure Chemical Industries (Tokyo, Japan). 2-(4-Diphenylphosphanyl-phenyl)-9-(1-hexyl-heptyl)-anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10-tetraone (Spy-LHP) and 2-(4-diphenylphosphanyl-phenyl)-9-(3,6,9,12-tetraoxatridecyl)-anthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10-tetraone (Liperfluo), 1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene (NOC7), and peroxynitrite were from Dojindo Laboratories (Kumamoto, Japan). Dulbecco's modified Eagle's medium (nutrient mixture F-12 Ham = 1:1 D-MEM/F-12) and SlowFade® Gold antifade reagent with DAPI were from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Hyclone (Logan, UT). Cumene hydroperoxide (Cumene-OOH) was from Alfa Aesar (Ward Hill, MA). 3-[4,5-Dimethylthiazol-2-yl]-2,5-di-phenyltetrazolium bromide (MTT) was from Nacalai Tesque (Kyoto, Japan).Synthesis of Liperfluo
Fluorometric analysis
A fluorescence spectrophotometer (JASCO, FP-6300) was used to measure fluorescence. The slit width for both excitation and emission was 2.5 nm. Relative quantum efficiencies of fluorescence for both Liperfluo and Liperfluo-Ox were obtained by comparing the area of the emission spectrum for the test sample with that of a solution of Spy-LHP-Ox (Φ ∼ 1) excited at the same wavelength. The quantum efficiencies of fluorescence were obtained from multiple measurements (n = 3) and calculated using the following equation:| Φsample = Φstandard × (Absstandard/Abssample) × (Σ[Fsample]/Σ[Fstandard]), |
where Abs and F denote absorbance and fluorescence intensity, respectively, and Σ[F] denotes the peak area of the fluorescence spectra calculated by summation of fluorescence intensities.
Cell culture
SH-SY5Y human neuroblastoma cells (American Type Culture Collection, Manassas, VA) were routinely maintained in D-MEM/F-12 medium containing 10% heat-inactivated fetal bovine serum and antibiotics (100 U mL−1 penicillin, 100 μg mL−1 streptomycin; Invitrogen) at 37 °C in 95% air and 5% CO2.Preparation of solutions of Spy-LHP and Liperfluo
Spy-LHP or Liperfluo was dissolved in CHCl3 at 10 mM and in DMSO at 1 mM concentrations. Liperfluo was completely dissolved in DMSO at 1 mM concentration, whereas Spy-LHP was not. Insoluble Spy-LHP in DMSO was removed by centrifugation, and a soluble fraction was used for experiments. The stock solution in CHCl3 and DMSO was diluted with serum-free medium before use for cell imaging. The final concentration of Spy-LHP or Liperfluo in culture medium was 20 μM. The final concentration of CHCl3 and DMSO was 0.2% and 2%, respectively.MTT assay
An MTT assay was conducted for the indicated periods, as described previously.21 SH-SY5Y cells were incubated in serum-free medium containing different concentrations of Liperfluo at 37 °C for 15 min, and then incubated in serum medium at 37 °C for 20 h. The cells were incubated with 0.5 mg mL−1 MTT at 37 °C for 4 h. Isopropyl alcohol containing 0.04 N HCl was added to the culture medium (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, by volume), and the mixture was agitated by pipette until the formazan was completely dissolved. The optical density of formazan was measured at 570 nm using an OPTImax Tunable microplate reader (Molecular Devices, Sunnyvale, CA).
2, by volume), and the mixture was agitated by pipette until the formazan was completely dissolved. The optical density of formazan was measured at 570 nm using an OPTImax Tunable microplate reader (Molecular Devices, Sunnyvale, CA).
LDH activity assay
A lactate dehydrogenase (LDH) activity assay was performed for the indicated periods, as described previously.21 Cells were incubated in serum-free medium containing different concentrations of Liperfluo at 37 °C for 15 min, and then incubated in serum medium at 37 °C for 24 h. LDH activity was measured by using iodotetrazolium chloride in the culture medium. The maximum LDH activity was determined by incubation of the cells with 1% Triton X-100. Data are expressed as a percentage of total LDH activity, after subtraction of background determined from the serum medium alone.Imaging procedures
SH-SY5Y cells were plated on a 6-well plate one day before imaging. Cells were incubated in serum-free medium containing Spy-LHP or Liperfluo at a final concentration of 20 μM, which was prepared as mentioned above, at 37 °C for 15 min. After removing the medium, cells were stimulated with either 6 mM AIPH or 100 μM Cumene-OOH at 37 °C for 2 h in serum-containing medium. After treatment, cells were fixed in 4% paraformaldehyde phosphate buffer solution. Changes in fluorescence were observed using an Olympus IX-71 epifluorescent microscope (Olympus, Tokyo, Japan). Nuclear staining was performed by SlowFade® Gold antifade reagent with DAPI.Confocal laser scanning imaging was performed with a Zeiss LSM 710 fluorescence microscope (Oberkochen, Germany). Argon lasers were used for excitation at 488 nm, and emissions were collected using appropriate band pass filters (515/530 nm).
Flow cytometric analysis
Cells were incubated in serum-free medium containing 20 μM Spy-LHP or Liperfluo at 37 °C for 15 min, and then incubated with either 6 mM AIPH or 100 μM Cumene-OOH at 37 °C for 2 h. Changes in fluorescence were measured using a BD FACSAriaTM II cell sorter (Becton, Dickinson and Company, Franklin Lakes, NJ) equipped with a 488 nm argon laser.References
- B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 3rd edn, 1999 Search PubMed.
- E. Niki, Y. Yoshida, Y. Saito and N. Noguchi, Biochem. Biophys. Res. Commun., 2005, 338, 668–676 CrossRef CAS.
- P. J. Marshall, M. A. Warso and W. E. Lands, Anal. Biochem., 1985, 145, 192–199 CrossRef CAS.
- M. A. Warso and W. E. Lands, J. Clin. Invest., 1985, 75, 667–671 CrossRef CAS.
- R. L. Heath and A. L. Tappel, Anal. Biochem., 1976, 76, 184–191 CrossRef CAS.
- Y. Yamamoto, M. H. Brodsky, J. C. Baker and B. N. Ames, Anal. Biochem., 1987, 160, 7–13 CrossRef CAS.
- C. P. LeBel, Chem. Res. Toxicol., 1992, 5, 227–231 CrossRef CAS.
- P. Wardman, M. J. Burkitt, K. B. Patel, A. Lawrence, C. M. Jones, S. A. Everett and B. Vojnovic, J. Fluoresc., 2002, 12, 65–68 CrossRef.
- H. Meguro, K. Akasaka and H. Ohrui, Methods Enzymol., 1990, 186, 157–161 CAS.
- Y. Okimoto, A. Watanabe, E. Niki, T. Yamashita and N. Noguchi, FEBS Lett., 2000, 474, 137–140 CrossRef CAS.
- M. Takahashi, M. Shibata and E. Niki, Free Radical Biol. Med., 2001, 31, 164–174 CrossRef CAS.
- Y. Okimoto, E. Warabi, Y. Wada, E. Niki, T. Kodama and N. Noguchi, Free Radical Biol. Med., 2003, 35, 576–585 CrossRef CAS.
- I. Matot, Y. Manevich, A. B. Al-Mehdi, C. Song and A. B. Fisher, Free Radical Biol. Med., 2003, 34, 785–790 CrossRef CAS.
- N. Soh, T. Ariyoshi, T. Fukaminato, H. Nakajima, K. Nakano and T. Imato, Org. Biomol. Chem., 2007, 5, 3762–3768 CAS.
- Y. Saito, Y. Yoshida and E. Niki, FEBS Lett., 2007, 581, 4349–4354 CrossRef CAS.
- M. Takahashi, M. Shibata and E. Niki, Free Radical Biol. Med., 2001, 31, 164–174 CrossRef CAS.
- A. Catala, Chem. Phys. Lipids, 2009, 157, 1–11 CrossRef CAS.
- H. Esterbauer, J. Gebicki, H. Puhl and G. Jurgens, Free Radical Biol. Med., 1992, 13, 341–390 CrossRef CAS.
- G. Takebe, J. Yarimizu, Y. Saito, T. Hayashi, H. Nakamura, J. Yodoi, S. Nagasawa and K. Takahashi, J. Biol. Chem., 2002, 277, 41254–41258 CrossRef CAS.
- W. Takabe, E. Warabi and N. Noguchi, Antioxid. Redox Signaling, 2011, 15, 1415–1426 CrossRef CAS.
- Y. Saito, K. Nishio, Y. Ogawa, T. Kinumi, Y. Yoshida, Y. Masuo and E. Niki, Free Radical Biol. Med., 2007, 42, 675–685 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
