Enhanced performance of core-shell-like structure Zr-doped CaCu3Ti4O12 ceramics prepared by a flame synthetic approach
W.L.
Li
ab,
Y.
Zhao
a,
Q.G.
Chi
c,
Z.G.
Zhang
b and
W.D.
Fei
*a
aKey Lab on Materials Behavior and Evaluation Technology in Space Environment, Department of Materials Physics and Chemistry, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: wdfei@hit.edu.cn; Fax: +86-451-86413908; Tel: +86-451-86413908
bCenter for Condensed Matter Science and Technology, Department of Physics, Harbin Institute of Technology, Harbin 150001, P. R. China.
cSchool of Applied Science, Key Laboratory of Engineering Dielectrics and its Application, Ministry of Education, Harbin University of Science and Technology, Harbin 150080, P. R. China.
First published on 3rd May 2012
Abstract
Zr-substituted CaCu3Ti4O12 ceramics were fabricated by a sol–gel flame synthetic approach. The influence of microstructure on the dielectric response of the ceramics has been investigated. It is found that CaCu3Ti4−xZrxO12 ceramics with a core-shell-like structure are obtained up to x = 0.05, and the thickness of the interphase region decreases with increasing Zr content. The maximum dielectric constant value is up to ∼6 × 104 for x = 0.05 at 1 kHz at room temperature. The high dielectric constant is connected to grain boundary effects, and the enhanced giant dielectric response with Zr substitution is attributed to a locally stretched lattice near Zr on the B-site and the resulting polarization switching of electric dipole moments.
Introduction
The CaCu3Ti4O12 (CCTO) compound, a complex cubic perovskite-like oxide, has recently attracted considerable attention due to its colossal dielectric constant in the order of magnitude 105 with weak temperature dependence between 100 K and 600 K in the frequency range from 100 Hz to 100 kHz.1–3 A great deal of work has been carried out in an attempt to understand the origin of these remarkable dielectric properties.4–6 It has now been established that the origin of such high permittivity values (>104) is of extrinsic polarization induced from an internal barrier layer structure. The internal barrier layer capacitance (IBLC) structure composed of conducting grains and insulating grain boundaries, supported by impedance spectroscopy results,7,8 has been proposed and widely accepted. Correspondingly, a nanoscale barrier layer capacitance (NBLC) model9 explained by polaron defects associated with stacking faults has been put forward to understand the origin of the dielectric property for CCTO in its various forms (single crystals, polycrystalline samples and films). Although the nature of the proposed barriers still has not been fully characterized, dielectric or capacitance spectroscopy analyses have shown that the grain boundaries indeed participate in the dielectric behavior.In addition, numerous reports are also focused on the dielectric behavior of doped CCTO ceramics.10,11 The 2% substitution of Mn for Cu suppresses dielectric constants by two orders of magnitude,12 and that TiO2 doping increases the internal potential barrier height in grain boundaries,13 indicating that the internal potential barrier structure can be changed by the doping process. Therefore, investigations into doped CCTO ceramics is expected to confirm the relationship between dielectric behavior and the barrier structure of CCTO.
Herein, we prepare CCTO ceramics with a core-shell-like structure via a sol–gel flame synthesis method. We show also here that partial substitution of Ti with Zr on the B-sites can lead to an anomalous increase in the dielectric constants of CCTO, and discuss the relationship between dielectric behavior and the grain boundaries layer structure variations of CCTO.
Experimental
CaCu3Ti4−xZrxO12(0 ≤ x ≤ 0.1) powders were synthesized by the sol–gel flame approach, similar to the traditional sol–gel route with the main difference of combusting the solution of precursors to synthesize powders. For the preparation of the precursor sol, calcium nitrate, copper nitrate, zirconium nitrate and titanium n-butoxide were used as starting materials, and 1-methoxyethanol was used as a solvent. At first, stoichiometric calcium nitrate, copper nitrate and zirconium nitrate were mixed and dissolved in 1-methoxyethanol. Secondly, under continual stirring, titanium n-butoxide was added to the solution, and the solution was stirred for 5 h to obtain a transparent precursor with a nominal composition of CaCu3Ti4−xZrxO12. Finally, by combusting the precursor sol, black CCTO powder was obtained. Then the powder was re-ground and pressed into disks of 10 mm diameter and 1 mm thickness. The disks were sintered in air at 1050 °C for 30 h and furnace-cooled to room temperature.The phase compositions of the CCTO ceramics were studied by using X-ray diffraction (XRD) on a Philips X'Pert diffractometer with Cu-Kα radiation generated at 40 kV and 40 mA. The microstructure and the morphology of the samples were characterized by a Hitachi S-4800 scanning electron microscope. Raman spectra measurements were performed on a Renishaw invia Confocal Raman Microscope, and an Ar+ laser (λ = 514.5 nm) was used as the excitation source. The chemical bonding and composition of the ceramics were investigated using X-ray photoelectron spectroscopy (PHI-ESCA5700). Both sides of the ceramics were deposited with platinum electrodes by magnetron sputtering. Room temperature dielectric properties of the samples were measured using an Agilent 4294A precision impedance analyzer.
Results and discussion
The XRD patterns of the CaCu3Ti4−xZrxO12 ceramics with different Zr contents, shown in Fig. 1, reveal a well crystallized cubic structure with Im3 space group, and no obvious secondary phase is detected up to x = 0.1. A slight increase of the lattice parameter with increasing Zr-content is attributed to the larger cationic radius of Zr compared to Ti.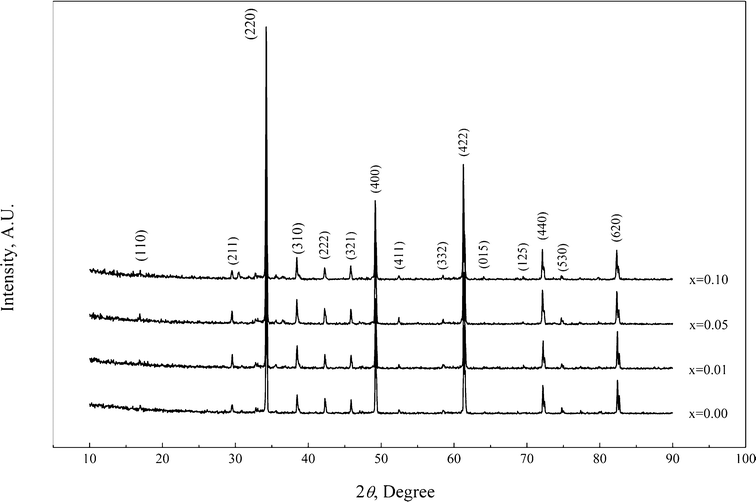 | ||
| Fig. 1 XRD patterns of the CaCu3Ti4−xZrxO12 ceramics with different Zr contents. | ||
Fig. 2 gives the scan electron microscopy (SEM) images of the surface of the CCTO ceramics with different Zr contents by sol–gel flame synthesis. The inset of the figure shows the microstructure of the local amplification of the SEM micrographs. A clear interface, appearing around most particles, depicts a perfect core-shell-like structure for the resultant particles. Grains with the size in the range 5–10 μm are observed in all CCTZO ceramics. The significant microstructure variation of CCTZO ceramics is visible with the increasing Zr content. The average grain size reduction, together with the decreased thickness of the interphase region, is accompanied by the increase of Zr content. The thickness of the interphase region is respectively about 750 nm and 500 nm for the pure CCTO ceramics and CCTZO ceramics with a Zr content of 0.01. Further increasing Zr content to 0.05, it decreases to 300 nm and disappears completely until the Zr content reaches 0.1.
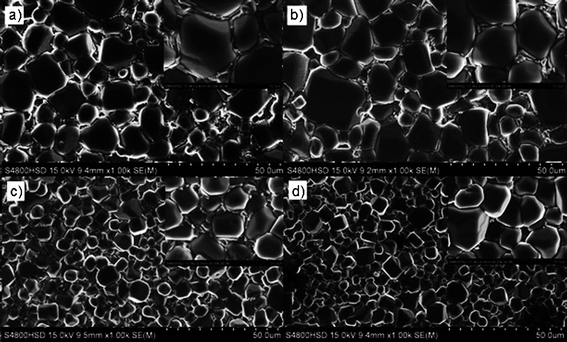 | ||
| Fig. 2 SEM images of the surface of the CaCu3Ti4−xZrxO12 ceramics with different Zr contents (a) x = 0, (b) x = 0.01, (c) x = 0.05, (d) x = 0.1. | ||
Energy dispersive spectroscopy (EDS) for the composition x = 0.05 (Fig. 3) further demonstrates that the interphase region is a Cu-rich area, and the interphase is a CuO-rich phase, which is in good accordance with previous result.14 The disappearance of the CuO-rich phase is caused by the incorporation of Cu cations into the grains.
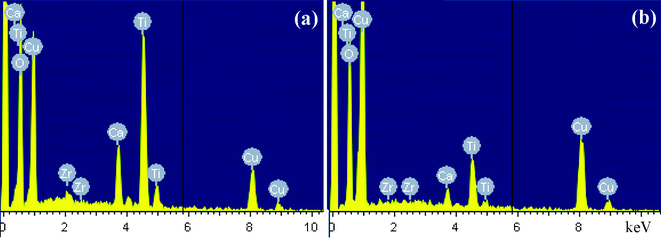 | ||
| Fig. 3 EDS of the CaCu3Ti3.95Zr0.05O12 ceramic, (a) grain, (b) grain boundary. | ||
Fig. 4 illustrates the frequency dependence of the dielectric constant and loss in CaCu3Ti4−xZrxO12 ceramics at room temperature. For clarity, the dielectric constant measured at 1 kHz is also given, as shown in Fig. 5. The dielectric constant increases with increasing x, and a giant dielectric constant plateau as high as ∼6 × 104 is achieved for x = 0.05 at room temperature and 1 kHz comparable with ∼3 × 104 for x = 0. However, the dielectric constant of x = 0.1 decreases to ∼3 × 104. The dielectric constant for x = 0.05 attains the highest value among all the samples over the measured frequency range.
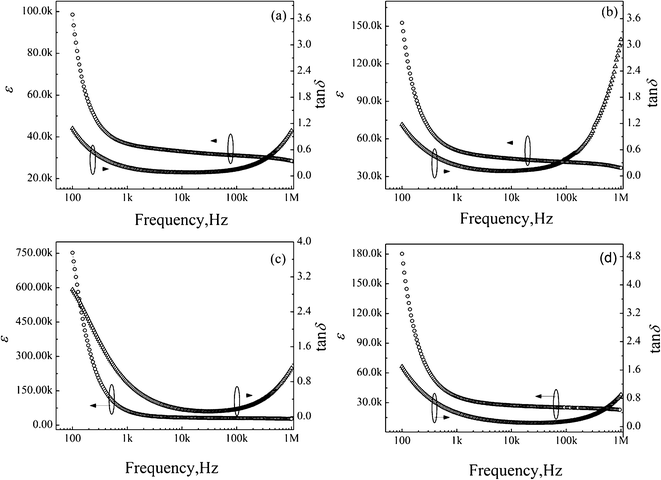 | ||
| Fig. 4 Frequency dependence of dielectric constant and loss in CaCu3Ti4−xZrxO12 ceramics measured at room temperature (a) x = 0, (b) x = 0.01, (c) x = 0.05, (d) x = 0.1. | ||
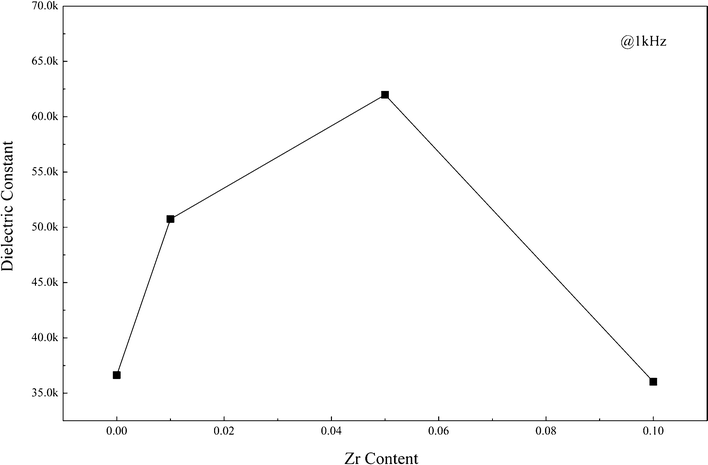 | ||
| Fig. 5 dielectric constant of CaCu3Ti4−xZrxO12 ceramics measured at 1 kHz and room temperature. | ||
Dielectric constants for the CCTZO samples decrease rapidly in the lower frequency range and subsequently remains nearly constant from 103 Hz to 106 Hz. The large dielectric constant at low frequency has been explained in terms of formation of barrier layers due to the conductivity difference between the grain and grain boundaries. The dielectric loss for the CCTZO samples is higher at low frequencies up to 1 kHz, and then remains at a relatively lower value (<0.2) up to 100 kHz.
Fig. 6 demonstrates the temperature dependence of the dielectric constant in CaCu3Ti3.95Zr0.05O12 for three fixed frequencies in the range of 100 Hz–10 kHz. There is an obvious dielectric relaxation at about 120 °C, which can be ascribed to Debye-like relaxation of Maxwell–Wagner polarization. The maximum dielectric constant value is up to ∼8 × 105 at 1 kHz.
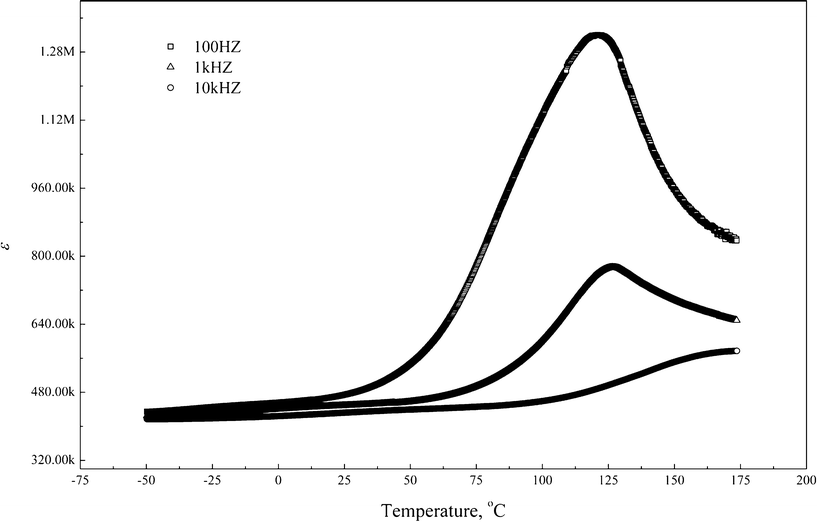 | ||
| Fig. 6 Temperature dependence of dielectric constant in CaCu3Ti3.95Zr0.05O12 ceramics. | ||
XPS analysis was carried out to investigate the oxidation states of polyvalent ions in CaCu3Ti4−xZrxO12 ceramics. Fig. 7 displays the XPS spectra of Ti 2p regions of CaCu3Ti4O12 and CaCu3Ti3.95Zr0.05O12. Both the Ti 2p3/2 peaks for x = 0 and x = 0.05 are symmetrical and can be fit into one peak, which shows that there exists only Ti4+ bonds at 458 eV in the CaCu3Ti4−xZrxO12 ceramics.
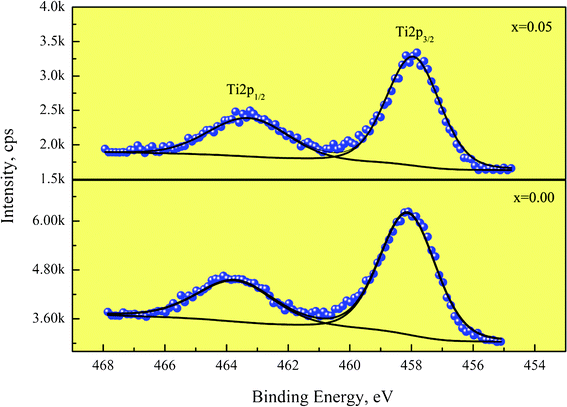 | ||
| Fig. 7 XPS spectra of the Ti 2p regions of CaCu3Ti4O12 and CaCu3Ti3.95Zr0.05O12 ceramics. | ||
In order to verify the probability of the polarization switching in the doped ceramics, Raman scattering measurements were employed, and the results are shown in Fig. 8. Fig. 8 shows the Raman spectra of the CaCu3Ti4−xZrxO12 ceramics, in which the three main peaks at 445, 510 and 575 cm−1 are easily identified in all the CCTO ceramics. Assignment of the Raman spectral features to the crystalline CCTO has been reported previously.15,16 The Raman lines at 445 and 510 cm−1 were associated with the Ag symmetry (TiO6, rotation-like) and 575 cm−1 of the Fg symmetry (O–Ti–O, anti-stretching).14 Our results are consistent with the previous Raman results for CCTO. However, with increasing Zr content, the major difference observed in the spectra is the intensity reduction of the Ag and Fg bands. The reduction of Ag (445 and 510 cm−1) bands, associated with rotation modes of the TiO6 octahedron, would impede the rotation of the octahedron. The reduction of Fg (575 cm−1) bands, associated with the O–Ti–O anti-stretching mode of the octahedron, would cause the off-center displacement of Ti ions within the TiO6 octahedron, which may result in the polarization switching in the doped ceramics.
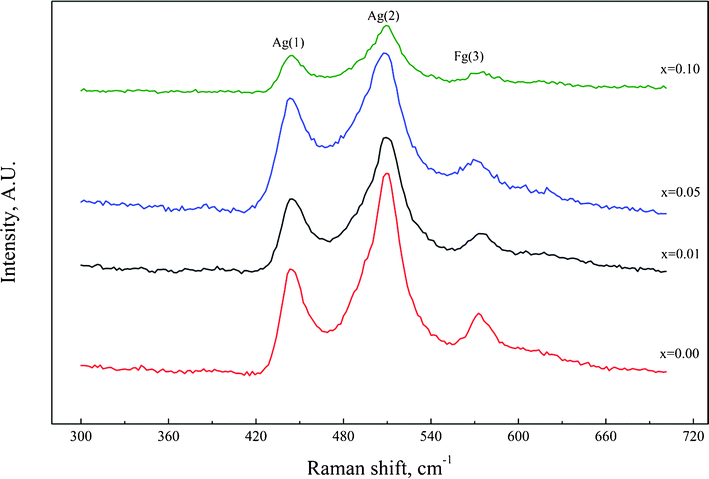 | ||
| Fig. 8 Raman spectra of the CaCu3Ti4−xZrxO12 ceramics. | ||
Some authors17–20 indicate that the high dielectric constants measured in CCTO samples can be generated by the IBLC mechanism and the modification of grain size may contribute to an enhanced dielectric response. However, these should not be the primary factors dominating the dielectric constant, more intrinsic origins should be taken into consideration.21,22
In our case, although the sintering time of CCTZO ceramics is 30 h, the grain size is still quite small (<10 μm) by the sol–gel flame synthetic approach. Moreover, the dielectric constant is not proportional to grain size. For the pure CaCu3Ti4O12 ceramics with core-shell-like structure, the high dielectric constant (∼3 × 104) corresponds with the IBLC effect. For the Zr-doped CaCu3Ti4O12 ceramics, the enhanced dielectric constant can be accounted for by the following two reasons. On the one hand, with increasing Zr content, the observed increase of the dielectric constant should be associated to the reduction of the Cu-rich intergranular phase thickness, as suggested by the IBLC model. On the other hand, a displacive ionic polarizability may come about because of the occurrence of local lattice stretches near Zr B-sites. The locally stretched lattice may result in a ‘rattling’ of some of the neighboring ions, causing the polarization switching.
Owing to the similar valence electron configuration of Zr and Ti cations, the increased dielectric constant in CCTZO may be unrelated to the electronic structure variation and the mixed-valent structure. Thus, we propose a displacive ionic polarizability in the unit cell bringing about initial ferroelectricity23 may be the origin of the high intrinsic bulk dielectric constant. The dielectric relaxation in CCTZO ceramics suggests the presence of intrinsic electric dipole moments and the intrinsic electric dipole moments are ordered to a certain extent. Under the conditions of doped strain, the polarization switching of the electric dipole moments becomes easier, resulting in the high dielectric constant (∼6 × 104).
Conclusion
We conclude that B-site Ti substitution by Zr can contribute to an anomalous increase in dielectric constant. A existence of a locally stretched lattice near the B-sites of Zr and the resulting polarization switching of the electric dipole moments may be regarded as the origin of the high intrinsic bulk dielectric constant.Acknowledgements
The project was supported by the Fundamental Research Funds for the Central Universities (Grant No. HIT.KLOF.2010002); China Postdoctoral Science Foundation; Postdoctoral Fund of Heilongjiang Province and Key Laboratory of Functional Materials Physics and Chemistry (Jilin Normal University), Ministry of Education.References
- M. A. Subramanian, D. Li, N. Duan, B. A. Reisner and A. W. Sleight, J. Solid State Chem., 2000, 151, 323 CrossRef CAS.
- C. C. Homes, T. Vogt, S. M. Shapiro, S. Wakimoto, M. A. Subramanian and A. P. Ramirez, Phys. Rev. B: Condens. Matter, 2003, 67, 092106 CrossRef.
- L. He, J. B. Neaton, M. H. Cohen, D. Vanderbilt and C. C. Homes, Phys. Rev. B: Condens. Matter, 2002, 65, 214112 CrossRef.
- S. V. Kalinin, J. Shin, G. M. Veith, A. P. Baddorf, M. V. Lobanov, H. Runge and M. Greenblatt, Appl. Phys. Lett., 2005, 86, 102902 CrossRef.
- S. Krohns, P. Lunkenheimer, S. G. Ebbinghaus and A. Loidl, Appl. Phys. Lett., 2007, 91, 022910 CrossRef.
- W. L. Li, W. T. Song, Y. Zhao, Q. G. Chi, N. Li, W. D. Fei and Z. G. Zhang, Mater. Chem. Phys., 2011, 129, 394 CrossRef CAS.
- D. C. Sinclair, T. B. Adams, F. D. Morrison and A. R. West, Appl. Phys. Lett., 2002, 80, 2153 CrossRef CAS.
- T. B. Adams, D. C. Sinclair and A. R. West, Adv. Mater., 2002, 14, 1321 CrossRef CAS.
- P. R. Bueno, R. Tararan, R. Parra, E. Joanni, M. A. Ramírez, W. C. Ribeiro, E. Longo and Jośe A. Varela, J. Phys. D: Appl. Phys., 2009, 42, 055404 CrossRef.
- G. Chiodelli, V. Massarotti, D. Capsoni, M. Bini, C. B. Azzoni, M. C. Mozzati and P. Lupotto, Solid State Commun., 2004, 132, 241 CrossRef CAS.
- R. K. Grubbs, E. L. Venturini, P. G. Clem, J. J. Richardson, B. A. Tuttle and G. A. Samara, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104111 CrossRef.
- J. N. Cai, Y. H. Lin, B. Cheng, C. W. Nan, J. L. He, Y. J. Wu and X. M. Chen, Appl. Phys. Lett., 2007, 91, 252905 CrossRef.
- Y. H. Lin, J. N. Cai, M. Li, C. W. Nan and J. L. He, Appl. Phys. Lett., 2006, 88, 172902 CrossRef.
- J. J. Romero, P. Leret, F. Rubio-Marcos, A. Quesada and J. F. Fernandez, J. Eur. Ceram. Soc., 2010, 30, 737 CrossRef CAS.
- N. Kolev, R. P. Bontchev, A. J. Jacobson, V. N. Popov, V. G. Hadjiev, A. P. Litvinchuk and M. N. Iliev, Phys. Rev. B: Condens. Matter, 2002, 66, 132102 CrossRef.
- D. Valim, A. G. Souza Filho, P. T. C. Freire, S. B. Fagan, A. P. Ayala, J. M. Filho, A. F. L. Almeida, P. B. A. Fechine, A. S. B. Sombra, J. S. Olsen and L. Gerward, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 132103 CrossRef.
- A. R. West, T. B. Adams, F. D. Morrison and D. C. Sinclair, J. Eur. Ceram. Soc., 2004, 24, 1439 CrossRef CAS.
- M. Li, A. Feteira, D. C. Sinclair and A. R. West, Appl. Phys. Lett., 2006, 88, 232903 CrossRef.
- M. Mitsugi, S. Asanuma, Y. Uesu, M. Fukunaga, W. Kobayashi and I. Terasaki, Appl. Phys. Lett., 2007, 90, 242904 CrossRef.
- G. Deng, T. Yamada and P. Muralt, Appl. Phys. Lett., 2007, 91, 202903 CrossRef.
- L. Ni and X.-M. Chen, J. Am. Ceram. Soc., 2010, 93, 184 CrossRef CAS.
- R. Schmidt and C. S. Derek, Chem. Mater., 2010, 22, 6 CrossRef CAS.
- M. C. Ferrarelli, D. C. Sinclair, A. R. West, H. A. Dabkowska, A. Dabkowski and G. M. Luke, J. Mater. Chem., 2009, 19, 5916 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
