Stripping voltammetry at chemically modified graphenes
Colin
Hong An Wong
and
Martin
Pumera
*
School of Physical and Mathematical Science, Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, Singapore. E-mail: pumera@ntu.edu.sg; Fax: +65 6791-1961
First published on 8th May 2012
Abstract
Several chemically modified graphene (CMG) materials were used to modify bare glassy carbon (GC) electrodes for the square wave anodic stripping voltammetry detection of cadmium ion concentration in aqueous solution, without the use of additives to amplify the detection signal. These CMGs included graphene oxide, graphite oxide, thermally reduced graphene oxide, chemically reduced graphene oxide and electrochemically reduced graphene oxide. The electrochemical performances of these modified electrodes were compared and the recently claimed advantages of using graphene materials to modify electrodes for the ASV detection of trace metal ions was thus challenged. Two CMG materials were proposed as suitable candidates for further investigations in their application towards real world sample analysis.
Introduction
The adverse effects of cadmium on the environment as well as living organisms are well documented in the literature, with decreased renal functionality, osteoporosis, pulmonary edema and even death among the more severe health risks observed in humans.1,2 Although the most dangerous form of exposure is in the form of fume and dust inhalation,3 ingestion of highly soluble cadmium compounds has also been shown to be extremely hazardous; a famous example of this was the mass cadmium poisoning case in Toyama, Japan, that arose from the pollution of rivers in the prefecture by mining companies.4 As such, the development of rapid, reliable, and portable on-site methods of Cd2+ ion detection is necessary. Conventional spectroscopic methods like inductively coupled plasma mass spectrometry and atomic absorption spectroscopy are unsuitable due to the high costs involved in operating the machinery and the bulkiness of the equipment.Electrochemical methods of trace metal ion analysis have relatively recently been garnering interest in the scientific community due to their low costs of operation, high sensitivity and portability. In particular, anodic stripping voltammetry (ASV) has come to be the main electrochemical method for trace metal detection due to its high sensitivity arising from the pre-concentration step.5 Initial efforts to further improve the sensitivity of measurements were centered around modifying the electrode surface with a metal film, as in the case of mercury film electrodes (MFEs) and bismuth film electrodes (BiFEs).6,7 Work involving the coating of the electrode surface with other metal-affinity compounds like dithizone and Nafion have also been noted.8,9
Modifications of the electrode with carbon-based materials (e.g. carbon paste,10 carbon nanotubes,11 calixarenes12etc.) for trace metal ion detection have also been reported. Graphene is a single-atom thick layer of planar sp2-hybridized carbon atoms closely arranged in a two-dimensional honeycomb structure that exhibits excellent electrical conductivity and mechanical properties, and is thus a promising and upcoming candidate for use as an electrochemical biosensor.13,14 This prompted the work by Li et al., in which they coated a GC electrode with a Nafion–graphene (Nafion–G) nanocomposite film and assessed its performance in Cd2+ detection in aqueous solution;15,29 this was expanded on in a study by Willemse et al. in which they used the Nafion–G electrode as an electrochemical detection platform for Zn2+, Cd2+, Pb2+ and Cu2+.16 Both works reported enhanced sensing capabilities of their Nafion–G electrodes and attributed this increased performance to the excellent electrical conductivity of graphene. Both works also used spiking of the electrolyte solution with Hg2+ to prepare in situ and ex situ MFEs. This would only serve to further complicate the interactions between Cd2+ ions in solution and the electrode surface; the precise reason behind the Nafion–G electrode's performance is thus not completely clear.
Chemically modified graphenes (CMGs) are a class of materials that possess functional groups bound to the surface of individual layers of carbon, as put forward by Ruoff and co-workers.17 Examples of these materials include graphite oxide, graphene oxide and reduced graphene oxides. A schematic of the derivation of CMGs from graphite is shown in Fig. 1.
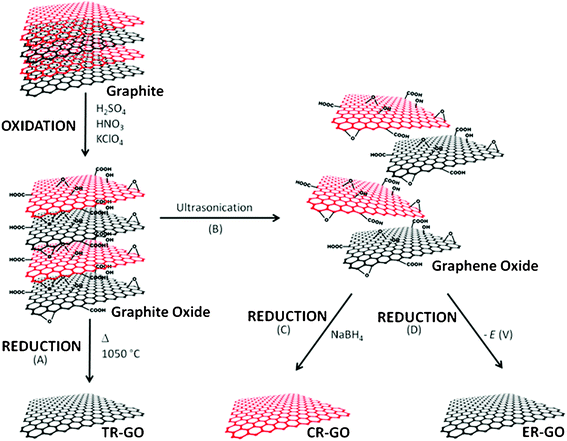 | ||
| Fig. 1 Schematic of the production of CMGs from graphite. Graphite was first oxidized to graphite oxide. Thermal reduction and exfoliation (A) of graphite oxide led directly to TR-GO. Graphite oxide was ultrasonicated (B) to generate graphene oxide, following which the chemical reduction (C) of graphene oxide yielded CR-GO. Alternatively, graphene oxide was electrochemically reduced (D) to afford ER-GO. | ||
To the best of our knowledge, to date there is no study on the ASV detection of Cd2+ ions in aqueous solution by modifying electrodes with CMG materials alone without the use of additives to amplify the peak arising from the Cd2+ ions. Herein, we report the first comparative assessment of five CMG materials: graphite oxide (GPO), graphene oxide (GO), chemically reduced graphene oxide (CR-GO), thermally reduced graphene oxide (TR-GO), and electrochemically reduced graphene oxide (ER-GO) in the electrochemical determination of trace Cd2+ ion concentration via square wave ASV (SWASV), including a control experiment involving a bare GC electrode.
Experimental section
Materials
Cadmium nitrate tetrahydrate was purchased from Alfa Aesar, Singapore. Graphite microparticles (> 20 μm), sodium borohydride, fuming nitric acid (> 90%), sulphuric acid (95–98%), potassium chlorate (98%), hydrochloric acid (37%), N,N-dimethylformamide, sodium acetate and glacial acetic acid (99.99%) were purchased from Sigma-Aldrich, Singapore. Deagglomerated alpha alumina powder (0.05 μm) was purchased from Struers, Singapore. The glassy carbon working electrode, silver/silver chloride reference electrode and platinum auxiliary electrode were obtained from CH Instruments, USA. The CMG materials were prepared and characterized in a previous study.18Apparatus
All voltammetric experiments were performed on a μAutolab type III electrochemical analyzer (Eco Chemie, The Netherlands) connected to a personal computer and controlled by General Purpose Electrochemical Systems Version 4.9 software (Eco Chemie). Electrochemical experiments were performed in a 20 mL electrochemical cell at room temperature by using a three-electrode configuration. A platinum electrode served as an auxiliary electrode, while an Ag/AgCl electrode served as a reference electrode. All the electrochemical potentials in this paper are stated versus the Ag/AgCl reference electrode.Procedures
Graphite oxide was prepared according to the Staudenmaier method.24 17.5 mL of sulphuric acid (95–98%) and 9 mL of nitric acid (fuming) were added to a reaction flask (round bottom) containing a magnetic stir bar. The mixture was cooled by immersion in an ice bath for 15 min. 1 g of graphite was then added to the mixture under vigorous stirring to avoid agglomeration and to obtain a homogeneous dispersion. While keeping the reaction flask in the ice bath, 11 g of potassium chlorate was slowly added to the mixture (over 15 min) in order to avoid a sudden increment in temperature and the formation of explosive chlorine dioxide gas. After the complete dissolution of potassium chlorate, the reaction flask was loosely capped to allow the evolution of gas and the mixture was stirred vigorously for 96 h at room temperature. On completion of the reaction, the mixture was poured into 1 L of deionized water and filtered. Graphite oxide was then redispersed and washed repeatedly in HCl (5%) solutions to remove sulphate ions and was finally washed with deionized water until a neutral pH of the filtrate was obtained. The graphite oxide slurry was then dried in a vacuum oven at 60 °C for 48 h before use.The graphene oxide to be used for the electrochemical investigations and characterizations was obtained by ultrasonication (37 kHz) for 1 h of a DMF dispersion of graphite oxide (1 mg mL−1).25
Thermally reduced graphene oxide was obtained by a thermal exfoliation/reduction of graphite oxide at 1050 °C. 0.2 g graphite oxide was placed in a porous quartz glass capsule connected to a magnetic manipulator inside a vacuum tight tube furnace with a controlled atmosphere. The application of the magnetic manipulator allowed us to create a temperature gradient over 1000 °C min−1. The sample was flushed with nitrogen by repeated evacuation of the tube furnace to remove any traces of oxygen and then quickly inserted by the magnetic manipulator to a preheated furnace and held in the furnace for 3 min. The flow of nitrogen during the exfoliation procedure was 1000 mL min−1 to remove the byproducts of the exfoliation procedure.26
Chemically reduced graphene oxide was obtained by mixing 10 mg of graphite oxide with 20 mL of an aqueous solution of NaBH4 (50 mM).27 The mixture was ultrasonicated (37 kHz) for 1 h. A reduction reaction is indicated by a color change from brown (graphene oxide) to black (CR-GO) during the sonication process. The CR-GO dispersion was then repeatedly washed with deionized water to remove NaBH4 residues and finally dried in a vacuum oven at 60 °C for 48 h.
Electrochemically reduced graphene oxide was obtained by applying a potential of −1.2 V (vs. Ag/AgCl) for 900 s to a graphene oxide modified GC electrode in a phosphate buffer solution (pH 7.2). The selection of the reducing potential was made in accordance to a prior cyclic voltammetric analysis. In this study the graphene oxide modified GC electrode exhibited a strong voltammetric reduction peak between −0.8 and −1.4 V with a maximum current at −1.2 V. This reduction peak corresponds to the reduction of graphene oxide on the electrode surface with the removal of the oxygen-containing groups28 and was selected for the reduction procedure.
Suspensions of the CMG materials in DMF were prepared with a concentration of 1 mg mL−1 with sonication for 30 min, with the exception of graphite oxide (GPO). Prior to each deposition onto a bare GC electrode, the suspension was sonicated for an additional 5 min, once again with the exception of GPO which was shaken by hand for 5 min. A 1 μL aliquot of the appropriate suspension was then deposited on the GC electrode and the solvent evaporated under a lamp for 30 min to provide the CMG-modified electrode.
Cadmium nitrate stock solution was prepared by dissolving cadmium nitrate tetrahydrate in water obtained from an ion-exchange system Milli-Q (Millipore), to a concentration of 10 mg L−1. Acetate buffer as background electrolyte was prepared with sodium acetate and acetic acid in Milli-Q water to a concentration of 0.1 M, pH 4.5.
SWASV measurements were carried out in the presence of dissolved oxygen in a 20 mL electrochemical cell containing 10 mL, 0.1 M acetate buffer (pH 4.5). A 60 s conditioning step at +0.6 V with stirring was performed before each measurement to remove any possible remaining reduced cadmium, followed by a deposition step of 120 s at a potential of −1.3 V at the CMG-modified electrode. The stirring was then stopped for a 15 s equilibration and the resulting voltammogram recorded with a square-wave potential scan from −1.3 V to −0.3 V, frequency 50 Hz, amplitude 20 mV and potential step 20 mV. Aliquots of the cadmium nitrate solution were added to the buffer solution before experimental measurements were taken. All electrodes were polished for 2 min with alumina slurry on a polishing pad, washed thoroughly with deionised water and then recoated with the appropriate CMG material between readings.
Results and discussion
The influence of modifying a bare GC electrode with five different CMG materials on the ASV detection of Cd2+ ions was investigated and the resulting voltammograms are shown in Fig. 2. Optimal experimental parameters such as step potential, amplitude, and frequency were adapted from a previous work by Kirgöz et al.19 For ease of representation of the data and to better appreciate the differences in response (particularly in the case of TR-GO), minor baseline corrections were performed on the raw voltammograms. Values were then obtained from these baseline-corrected voltammograms to construct suitable calibration plots for peak current value against Cd2+ concentration. As can be seen from Fig. 2, modification of the electrode surface with CMG materials does offer increased sensitivity in terms of the increase in peak current height over the bare GC electrode, for all five CMG materials tested. In addition, the ASV responses of the CMG modified electrodes seem to be split into two groups: the oxides (GPO, GO) and the reduced graphene oxides (CR-GO, TR-GO, ER-GO). The calibration plots obtained for each CMG material were linear with R2 values greater than 0.96. Relevant information from the calibration plots are detailed in Table 1 (the best values are in bold). The bare GC electrode gave a highly linear calibration plot of [Cd2+]; however, the sensitivity (as depicted from the slope) was the lowest amongst all the materials tested. In contrast, the GPO modified electrode showed a more than twofold increase in sensitivity but suffered from more reproducibility issues (average relative standard deviation, RSD, of 22.51%). Modification with GO gave approximately similar results, albeit with a slightly lower sensitivity but also lower RSD values. The CR-GO and TR-GO modified electrodes behaved similarly in terms of both the reproducibility and sensitivity (average RSD ∼10%, slope ∼0.130), while the ER-GO modified electrode performed markedly poorer in reproducibility and linearity despite all three reduced graphene oxide materials having highly similar peak height values at [Cd2+] = 20–25 μg L−1. A graphical representation of these comparisons is depicted in Fig. 3.![Baseline-corrected square-wave stripping voltammograms for increasing concentrations of Cd2+ in 5 μg L−1 steps for (A) bare GC, (B) GPO, (C) GO, (D) CR-GO, (E) TR-GO, and (F) ER-GO modified electrodes. Blank voltammograms also shown. Calibration plots over [Cd2+] ranging from 5–25 μg L−1 are inset with error bars. Background electrolyte 0.1 M acetate buffer (pH 4.5); square-wave voltammetric scan with frequency = 50 Hz, potential step = 20 mV, amplitude of 20 mV; deposition potential of −1.3 V for 120 s.](/image/article/2012/RA/c2ra20431b/c2ra20431b-f2.gif) | ||
| Fig. 2 Baseline-corrected square-wave stripping voltammograms for increasing concentrations of Cd2+ in 5 μg L−1 steps for (A) bare GC, (B) GPO, (C) GO, (D) CR-GO, (E) TR-GO, and (F) ER-GO modified electrodes. Blank voltammograms also shown. Calibration plots over [Cd2+] ranging from 5–25 μg L−1 are inset with error bars. Background electrolyte 0.1 M acetate buffer (pH 4.5); square-wave voltammetric scan with frequency = 50 Hz, potential step = 20 mV, amplitude of 20 mV; deposition potential of −1.3 V for 120 s. | ||
![Overlays of the calibration plots for CMG modified electrodes used in the ASV analysis of [Cd2+].](/image/article/2012/RA/c2ra20431b/c2ra20431b-f3.gif) | ||
| Fig. 3 Overlays of the calibration plots for CMG modified electrodes used in the ASV analysis of [Cd2+]. | ||
| Electrode | R2 value | Min %RSD | Max %RSD | Avg. %RSD | Slope |
|---|---|---|---|---|---|
| GC | 0.9852 | 5.62 | 14.61 | 11.17 | 0.076 |
| GPO | 0.9827 | 15.64 | 35.57 | 22.51 | 0.197 |
| GO | 0.9777 | 6.08 | 21.50 | 15.67 | 0.162 |
| CR-GO | 0.9796 | 6.64 | 11.17 | 9.92 | 0.124 |
| TR-GO | 0.9852 | 7.00 | 19.77 | 10.80 | 0.135 |
| ER-GO | 0.9634 | 13.71 | 35.99 | 20.05 | 0.147 |
From the roughly similar stripping responses for the reduced graphene oxides it can be inferred that the most likely factor influencing the enhancement in sensitivity (as compared to bare GC) is the increased specific surface area resulting from the deposition of a CMG film on the electrode surface, and is hence a physical cause instead of any novel electrochemical properties possessed by graphene and its related materials.20 Additionally, since pure GPO and GO themselves are non-conducting, the higher responses from the oxide material modified electrodes imply that any conductivity benefit gleaned from using graphene in ASV is far outweighed by the presence of oxygen-containing groups on GPO and GO. This is in contrast to recent claims of the high conductivity of graphene sheets being greatly advantageous towards trace metal analysis via ASV.21 Surfaces with anionic and/or oxygen-containing groups have been shown to have increased metal cation adsorption possibly due to ligand coordinating effects, but the precise mechanism is still not fully understood.22,23
Full width at half maximum (FWHM) values are important in practical applications of ASV, as real world samples for trace metal analysis will contain multiple ion peaks that may eclipse each other if the oxidation potentials are close. Hence, narrow FWHM ranges are desirable to ensure good peak resolution and accurate analyte concentration determination. FWHM values were consequently obtained from the voltammograms and a summary for each CMG-modified electrode are shown in Table 2. Improvements in the FWHM values were noted for all five CMG-modified electrodes over the bare GC electrode: FWHM values were approximately halved after modification with the sole exception of the TR-GO modified electrode, in which a decrease of 23.6% was observed. This implies that CMG-modified electrodes are generally more suited towards real world sample analysis than the bare GC electrode, since the bare GC would be more susceptible to peak eclipsing. The half maximum width difference between the materials as well as the difference in response in general can be explained by considering the different strength of the interaction of different oxygen containing groups with Cd2+ ion.
| Electrode | GC | GPO | GO | CR-GO | TR-GO | ER-GO |
|---|---|---|---|---|---|---|
| Average FWHM (V) | 0.165 | 0.090 | 0.076 | 0.076 | 0.126 | 0.079 |
With regards to the limits of detection, the CMG-modified electrodes gave the following results (values in μg L−1)—GPO: 2.41; GO: 1.08; CR-GO: 1.56; TR-GO: 2.78; ER-GO: 5.70. This data shows that GO and CR-GO modified electrodes have the lowest limits of detection out of all the CMG materials tested, and as such are more suitable candidates for Cd2+ detection in aqueous systems.
For trace metal analysis in real world samples via ASV it can be seen that while GPO-modified electrodes would potentially offer the best sensitivity, the large %RSD values would impair the electrode's accuracy, which is of utmost importance in determining an experimental methods’ applicability to sample analysis. Taking into account the cumulative effects of the factors considered in the experiment, both GO and CR-GO modified electrodes offer the best combination of sensitivity, reproducibility, detection limits and FWHM values compared to the rest of the materials, with GO-modified electrodes being more sensitive but suffering from lower reproducibility. As a result, selection of either material for the modification of electrodes would depend on whether sensitivity or reproducibility is deemed more important. More detailed investigations are currently in progress to assess these CMG materials in their application towards the ASV detection of other metal ions in solution and the determination of metal ion concentration in real world samples.
Conclusion
We have assessed the electrochemical responses of five CMG materials in trace [Cd2+] detection via ASV by modifying a GC electrode and comparing their performances with each other and that of a bare GC electrode. We showed that all CMG-modified electrodes offer advantages in various forms like increased sensitivity, lower detection limits, and higher peak resolution but have generally less reproducible data. We also demonstrated that using graphene materials for the ASV analysis of [Cd2+] was on par with or offered a lower performance compared to their oxidized counterparts. GO and CR-GO showed the best combination of increased performance over bare GC electrodes. Further research is necessary to fully assess the feasibility of CMG-modified electrodes in real world multi-ion sample ASV analysis and is currently underway.Acknowledgements
We wish to acknowledge NAP start-up grant (NTU) for financial support. We also wish to acknowledge the funding support for this project from Nanyang Technological University under the Undergraduate Research Experience on CAmpus (URECA) programme.References
- A. W. Hayes, Principles and Methods of Toxicology, CRC Press, Philadelphia, 2007 Search PubMed.
- L. Friberg, Annu. Rev. Public Health, 1983, 4, 367–367 CrossRef CAS.
- R. E. Kirk and D. F. Othmer, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, New York, 4th edn, 1994 Search PubMed.
- K. Nogawa, E. Kobayashi, Y. Okubo and Y. Suwazono, BioMetals, 2004, 17, 581–587 CrossRef CAS.
- J. Wang, Stripping Analysis: Principles, Instrumentation and Applications, VCH Publishers, Deerfield Beach, 1985 Search PubMed.
- A. Economou and P.R. Fielden, Analyst, 2003, 128, 205–213 CAS.
- J. Wang, J. Lu, S. B. Hocevar and B. Ogorevc, Electroanalysis, 2001, 13, 13–16 CrossRef CAS.
- I. Palchetti, C. A. Upjohn, P. F. Turner and M. Mascini, Anal. Lett., 2000, 33, 1231–1246 CrossRef CAS.
- A. Merkoçi, M. Vasjari, E. Fabregas and S. Alegret, Microchim. Acta, 2000, 135, 29–33 CrossRef.
- S. S. Huang, Y. D. Cheng, B. F. Li and G. D. Liu, Microchim. Acta, 1998, 130, 97–101 CrossRef CAS.
- K. Wu, S. Hu, J. Fei and W. Bai, Anal. Chim. Acta, 2003, 489, 215–221 CrossRef CAS.
- K. C. Honeychurch, J. P. Hart, D. C. Cowell and D. W. M. Arrigan, Electroanalysis, 2002, 14, 177–185 CrossRef CAS.
- Y. Wang, Y. M. Li, L. H. Tang, J. Lu and J. H. Li, Electrochem. Commun., 2009, 11, 846–849 CrossRef.
- J. Lu, I. Do, L. T. Drzal, R. M. Worden and I. Lee, ACS Nano, 2008, 2, 1825–1832 CrossRef CAS.
- J. Li, S. Guo, Y. Zhai and E. Wang, Electrochem. Commun., 2009, 11, 1085–1088 CrossRef CAS.
- C. M. Willemse, K. Tlhomelang, N. Jahed, P. G. Baker and E. I. Iwuoha, Sensors, 2011, 11, 3970–3987 CrossRef CAS.
- D. R. Dreyer, R. S. Ruoff and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 9336–9344 CrossRef CAS.
- A. Ambrosi, A. Bonanni, Z. Sofer and M. Pumera, Chem.–Eur. J., 2011, 17, 10763–10770 CrossRef CAS.
- Ü. A. Kirgöz, S. Marin, M. Pumera, A. Merkoçi and S. Alegret, Electroanalysis, 2005, 17, 881–886 CrossRef.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS.
- J. Li, S. Guo, Y. Zhai and E. Wang, Anal. Chim. Acta, 2009, 649, 196–201 CrossRef CAS.
- D. A. Dzombak and F. M. M. Morel, J. Hydraul. Eng., 1987, 113, 430–475 CrossRef.
- Y.-H. Li, S. Wang, Z. Luan, J. Ding, C. Xu and D. Wu, Carbon, 2003, 41, 1057–1062 CrossRef CAS.
- L. Staudenmaier, Ber. Dtsch. Chem. Ges., 1898, 31, 1481 CrossRef CAS.
- J. I. Paredes, S. Villar-Rodil, A. Martinez-Alonso and J. M. D. Tascon, Langmuir, 2008, 24, 10560 CrossRef CAS.
- M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud'homme and I. A. Aksay, Chem. Mater., 2007, 19, 4396 CrossRef CAS.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park, I. S. Jung, M. H. Jin, H. K. Jeong, J. M. Kim, J. Y. Choi and Y. H. Lee, Adv. Funct. Mater., 2009, 19, 1987 CrossRef CAS.
- M. Zhou, Y. L. Wang, Y. M. Zhai, J. F. Zhai, W. Ren, F. A. Wang and S. J. Dong, Chem.–Eur. J., 2009, 15, 6116 CrossRef CAS.
- J. Li, S. Guo, Y. Zhai and E. Wang, Anal. Chim. Acta, 2009, 649, 196 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
