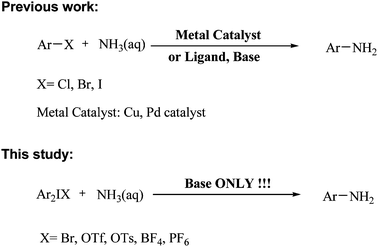
Simple and efficient amination of diaryliodonium salts with aqueous ammonia in water without metal-catalyst†
Jian
Li
*a and
Li
Liu
b
aSchool of Pharmaceutical Engineering & Life Sciences, Chang Zhou University, Changzhou, 213164, P. R. China. E-mail: lijianchem@gmail.com
bAnalytical center, Chang Zhou University, Changzhou, 213164, P. R. China
First published on 11th September 2012
Abstract
A simple and effective amination of diaryliodonium salts with aqueous ammonia has been developed, giving anilines in high yields without metal-catalyst and ligand.
Anilines are important building blocks for constructing natural products, pharmaceutical and medicinal compounds, as well as in polymers and materials.1 Although many methods are available, the discovery of new and improved methodologies is still of interest.1,2 Over the last decades, impressive progress has been made in transition-metal-catalyzed C–N bond formation, in particularly, the palladium-catalyzed Buchwald–Hartwig reactions3 and copper-catalyzed Ullman-type transformations.4 Ammonia is used as one of the most attractive sources of nitrogen for the synthesis of anilines because of its low cost and wide availability.1 The traditional methods for the preparation of anilines by the direct coupling of aryl halides with ammonia were less practical due to the required high pressure, high temperature, long reaction time and the undesirable diarylamine by-products.5 In recent years, palladium and copper-catalysis of the aryl halide and ammonia couplings affording the anilines under mild conditions have been reported.6,7 Among these, Buchwald's and Hartwig's groups reported the palladium-catalyzed selective coupling reactions of aryl halides with ammonia.6a–6e In 2008, Chang and Zhao developed a highly efficient copper-catalyzed procedure for the direct coupling reaction between aryl iodides and NH3 (aq.) in the presence of L-proline.7a,7b In 2009, Xia and Taillefer uncovered a copper/diketone catalytic system for the direct amination of both aryl iodides and bromides in aqueous ammonia.7e,f Lately, Wolf utilized Cu2O as a catalyst to perform the amination of aryl halides in NMP/water media.7g Darcel described an efficient catalytic system based on Fe2O3/CuI for the amination of aryl iodides by using aqueous ammonia as a nitrogen source.7h Very recently, Xu developed a protocol for aniline synthesis involving the CuI-nanoparticles-catalyzed coupling of aryl iodides with ammonia at room temperature.7i
Unfortunately, all the above catalytic reactions are inefficient in neat aqueous medium in the absence of a metal-catalyst. There are rare successful examples of the direct coupling with aqueous ammonia under mild conditions without metal-catalyzed. It is highly desirable to develop new efficient methods to further improve the efficiency and generality of the preparation of primary aromatic amines via metal-free process.
The use of diaryliodonium salts has recently gained considerable attention in organic synthesis.8 As part of our ongoing interest in developing application of diaryliodonium salts,9 we herein wish to report a simple, efficient method for preparation of primary aromatic amines without a metal-catalyst (Scheme 1).
At the outset of our studies, a set of experiments was carried out using diphenyliodonium salt 1a and aqueous ammonia as model substrates. We tested various reaction conditions for the amination of diphenyliodonium salt (Table 1).
| Entry | Base | X | Yieldb (%) |
|---|---|---|---|
| a Diaryliodonium triflate salt (0.4 mmol) and 25–28% aqueous ammonia (1 mL) were mixed and heating to 80 °C. b GC yield. c Reaction temperature is 90 °C. d CuI (10 mol %) was added. e CuI (10 mol %) was added and reaction was proceed at room temperature. | |||
| 1 | — | OTs | 5 |
| 2 | NaOH | OTs | 67 |
| 3c | NaOH | OTs | 35 |
| 4 | CuI | OTs | 23 |
| 5d | NaOH(CuI) | OTs | 59 |
| 6e | NaOH(CuI) | OTs | 42 |
| 7 | NaOH | BF4 | 81 |
| 8 | NaOH | PF6 | 67 |
| 9 | NaOH | Br | 61 |
| 10 | NaOH | OTf | 98 |
| 11 | KOH | OTf | 91 |
| 12 | Na2CO3 | OTf | 6 |
| 13 | K2CO3 | OTf | 2 |
| 14 | Cs2CO3 | OTf | 2 |
| 15 | NaOAc | OTf | 7 |
| 16 | K3PO4 | OTf | 11 |
| 17 | NaOEt | OTf | 5 |
First, diphenyliodonium p-methylbenzenesulfonate salt was chosen as the test substrate with aqueous ammonia, these two coupling partners were reacted in H2O at 80 °C without the presence of metal-catalyst and base, and only 5% yield of aniline was obtained (entry 1, Table 1). When 2 equiv. of NaOH was employed, we are pleased to find that the reaction occurred to afford 2a in 67% yield (entry 2, Table 1).‡
It is noteworthy that a low yield (35%) was obtained when the reaction was carried out at higher temperature (entry 3, Table 1). The low yield is due to the loss of ammonia at high temperatures; on the other hand, a side reaction has occurred under the harsh reaction condition, with 37% yield of diphenyl ether found from GC-MS; the process is shown in Scheme 2.
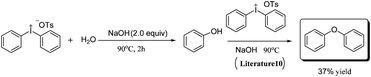 | ||
| Scheme 2 Side reaction under high temperature.10 | ||
As mentioned previously, a copper-catalyst can be a good catalyst for amination with ammonia, so CuI was used as the catalyst in this reaction under the same conditions without NaOH; 23% yield of the target product was found, from GC-MS we find the major product was diphenyl, which was the self-coupling product from diphenyliodonium 1a. The yield was increased to 59% in CuI/NaOH catalyzed system and 42% yield of target product was obtained when the reaction occurred at room temperature under the similar condition (entries 5 and 6, Table 1).
Further studies were thus carried out about the influence of different diphenyliodonium anions, which showed that satisfactory results could be obtained with diaryliodonium tetrafluoroborates, hexafluorophosphates, bromide (entries 7–10, Table 1). Gratifyingly, significant improvement was achieved by the use of diphenyliodonium triflate, which had better solubility in water and weak nucleophilicity, resulting in clean formation of the product in 98% yield (entry 10).
Base is another important factor to affect the catalysis. Among various bases examined, Na2CO3, K2CO3, Cs2CO3, NaOAc, K3PO4 and NaOEt were all poorly effective for the catalysis, but the use of KOH led to excellent yield (entry 10, Table 1). It seems that the reaction needs to be promoted with a stronger base. Thus, NaOH was selected as the base in the following studies.
With the optimized reaction conditions in hand, we probed the scope of amination with different diaryliodonium triflates employing 2 equiv. of NaOH in H2O at 80 °C and the results are summarized in Table 2.
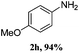
The reaction scope was subsequently explored by using various asymmetrical diaryl iodonium salts 1 which were prepared with appropriate iodoarene and mesitylene.11 The electronic effects on the reactivity were limited. The coupling of aqueous ammonia with most electron-donating substituents worked equally well as those with electron-withdrawing substituents, affording the desired products 2a–p with good to excellent yields in 2 h.
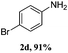
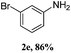
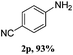
Steric bulk in the meta-position was very well tolerated, as demonstrated by amination with meta-bromo diaryl iodonium salt to yield a highly congested product 2e. However, steric hindrance of ortho-substituents resulted in a lower reactivity of the corresponding diaryl iodonium salts to afford lower yield (2g, Table 2). Similarly, the isolated yield is only 71% for 2,4,6-trimethylbenzenamine (2j, Table 2). Interestingly, fused ring 1-naphthalene iodonium salts can also be successfully converted to 1-naphthalenamine with a yield of 90% (2q).
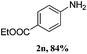
Furthermore, it must be pointed out that the lower yields of 2m and 2n did not indicate that the ester groups substituents had lower reactivity with ammonia; from GC-MS the carboxyl group product 4-aminobenzoic acid was found which was hydrolysed from target products under strong basic conditions at 80 °C.
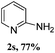
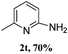
The present reaction was also examined with various heterocyclic diaryliodonium salts, such as pyridine and thiophene (Table 3). Thus, phenyl (pyridin-3-yl)iodonium salt underwent an amination reaction with aqueous ammonia providing 3-amino pyridin 2r in 81% yield. Substituted pyridine diaryliodonium salts also efficiently reacted with ammonia giving the corresponding products 2t and 2u in 70% and 63% yields, respectively. Similarly, 2-amino thiophene (2v) was isolated under our standard conditions, which is a privileged scaffold.
Diaryliodonium salts, owing to their highly electron-deficient nature and excellent leaving-group ability, they serve as versatile arylating agents with a variety of nucleophiles, including ammonia and other sources of nitrogen. Finally, in order to verify the reactivity of diaryliodonium salts in amination, an investigation of the amination of diaryliodonium triflate salt under the previously optimized conditions with another source of nitrogen, ammonium chloride, was carried out. To our delight, the results exceeded our expectations; 61% yield of phenylamine was obtained and another byproduct was phenol in 30% yield (Scheme 3).
 | ||
| Scheme 3 Preparation of anilines with ammonium chloride. | ||
In conclusion, a simple and effective amination of diaryliodonium salts with aqueous ammonia has been developed under basic condition at 80 °C, good to excellent yields are obtained without the use of a palladium- or copper-catalyst and ligand in 2 h, with yields up to 98%. There are rare successful examples of the direct coupling with aqueous ammonia under mild conditions without a metal-catalyst, our catalyzed system is one of them. Overall, we believe this environmentally benign system will find wide application in organic synthesis. Current studies are focused on further exploration of the substrate scope and synthetic utility of this catalyzed system.
The authors acknowledge grants from the Foundation of Changzhou Municipal Bureau of Science and Technology (No. KYZ1102100C) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- (a) R. C. Larockin, Comprehensive Organic Transformations: A Guide to Functional Group Preparation, VCH, NewYork, 1989 Search PubMed; (b) K. Weissermel and H. J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 1997 Search PubMed; (c) S. A. Lawrence, Amines: Synthesis Properties and Applications, Cambridge University Press, Cambridge, 2004 Search PubMed.
- (a) M. Negwer, Organic Drugs and Their Synonyms, 7th ed., Akademie Verlag, Berlin, 1994 Search PubMed; (b) B. Schlummer and U. Scholz, Adv. Synth. Catal., 2004, 346, 4052 CrossRef; (c) D. M. Roundhill, Chem. Rev., 1992, 92, 1 CrossRef CAS.
- Recent reviews of palladium-catalyzed C–N coupling reactions: (a) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS; (b) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS.
- Reviews of copper-catalyzed C–N coupling reactions: (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (b) W. Deng, L. Liu and Q.-X. Guo, Chin. J. Org. Chem., 2004, 24, 150 CAS; (c) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS; (d) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2008, 47, 3096 CrossRef CAS; (e) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS; (f) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS.
- (a) H. H. Wenk, M. Winkler and W. Sander, Angew. Chem., Int. Ed., 2003, 42, 502 CrossRef CAS; (b) H. Pellissier and M. Santelli, Tetrahedron, 2003, 59, 701 CrossRef CAS; (c) C. Xie and Y. Zhang, Org. Lett., 2007, 9, 781 CrossRef CAS.
- Palladium-catalyzed formation of aromatic amines: (a) Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 10028 CrossRef CAS; (b) D. S. Surry and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 10354 CrossRef CAS; (c) D. Y. Lee and J. F. Hartwig, Org. Lett., 2005, 7, 1169 CrossRef CAS; (d) X. H. Huang, K. W. Anderson, D. Zim, L. Jiang, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 6653 CrossRef CAS; (e) M. Willis, Angew. Chem., 2007, 119, 3470 CrossRef; (f) M. Willis, Angew. Chem., Int. Ed., 2007, 46, 3402 CrossRef CAS.
- Copper-catalyzed formation of aromatic amines: (a) J. Kim and S. Chang, Chem. Commun., 2008, 3052 RSC; (b) X. Gao, H. Fu, R. Qiao, Y. Jiang and Y. Zhao, J. Org. Chem., 2008, 73, 6864 CrossRef CAS; (c) R. Ntaganda, B. Dhud-shia, C. L. B. Macdonald and A. Thadani, Chem. Commun., 2008, 6200 RSC; (d) S. Gaillard, M. K. Elmkaddem, C. Fischmeister, C. M. Thomas and J.-L. Renaud, Tetrahedron Lett., 2008, 49, 3471 CrossRef CAS; (e) N. Xia and M. Taillefer, Angew. Chem., 2009, 121, 343 CrossRef; (f) N. Xia and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 337 CrossRef CAS; (g) H. Xu and C. Wolf, Chem. Commun., 2009, 3035 RSC; (h) X.-F. Wu and C. Darcel, Eur. J. Org. Chem., 2009, 4753 CrossRef CAS; (i) H.-J. Xu, Y.-F. Liang, Z.-Y. Cai, H.-X. Qi, C.-Y. Yang and Y.-S. Feng, J. Org. Chem., 2011, 76, 2296 CrossRef CAS.
- For reviews on diaryliodonium salts, see: (a) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS; (b) A. Varvoglis, The Organic Chemistry of Poly coordinated Iodine; VCH Publishers: New York, 1992 Search PubMed; (c) V. V. Grushin, Chem. Soc. Rev., 2000, 29, 315 RSC; (d) J. M. Becht and C. L. Drian, Org. Lett., 2008, 10, 3161 CrossRef CAS; (e) X. Qu, P. Sun, T. Li and J. Mao, Adv. Synth. Catal., 2011, 353, 1061 CrossRef CAS; (f) J. Aydin, J. M. Larsson, N. Selander and K. J. Szabó, Org. Lett., 2009, 11, 2852 CrossRef CAS.
- J. Li, L. Liu, Y.-Y. Zhou and S.-N. Xu, RSC Adv., 2012, 2, 3207 RSC.
- N. Jalalian, E. E. Ishikawa, L. F. Silva Jr and B. Olofsson, Org. Lett., 2011, 13, 1552 CrossRef CAS.
- (a) J.-H. Chun, S. Lu and V. W. Pike, Eur. J. Org. Chem., 2011, 4439 CrossRef CAS; (b) M. Bielawski, M. Zhu and B. Olofsson, Adv. Synth. Catal., 2007, 349, 2610 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: experimental details and the characterization data for all compounds. See DOI: 10.1039/c2ra22046f |
| ‡ Representative procedure: under nitrogen atmosphere, a solution of 25–28% aqueous ammonia (1 mL) and diaryliodonium triflate salt (172.4 mg, 0.40 mmol) was treated with NaOH (32 mg, 0.80 mmol) at 80 °C for 2 h. The temperature was allowed to cool slowly, HCl (3 mL, 1 M) and ethyl acetate (5 mL) were added to the reaction mixture separately, the organic layer was separated and concentrated, and iodobenzene was obtained. Aqueous phase was alkalinized by NaOH (1 M) and extracted with ethyl acetate (5 mL); the organic layer was dried and concentrated to provide 2a (36.5 mg) in 98% yield. |
| This journal is © The Royal Society of Chemistry 2012 |