A green process for the epoxidation of dicyclopentadiene with aqueous H2O2 over highly efficient and stable HPW-NH2-SBA-15
Ruihua
Gao
ab,
Quanjing
Zhu
a,
Wei-Lin
Dai
*a and
Kangnian
Fan
a
aShanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, Shanghai 200433, P.R.China. E-mail: wldai@fudan.edu.cn; Fax: +86-21-55665701
bResearch Center of Nano Science and Technology, Shanghai University, Shanghai 200444, P.R. China
First published on 6th June 2012
Abstract
Dicyclopentadiene dioxide (2) was synthesized using an economic and green reaction by the direct oxidation of dicyclopentadiene (DCPD) with aqueous H2O2 over tungstic acid and aminopropyl-immobilized phosphotungstic acid on SBA-15, which was successfully obtained by the immobilization of the supported heteropolyacid (HPW) on the surface of the ordered mesoporous silica, SBA-15, by means of chemical bonding to aminosilane groups. The as-obtained materials were characterized by N2 sorption, transmission electron microscopy (TEM), X-ray diffraction (XRD), 31P-magic angle spinning (MAS) NMR and Raman spectroscopy. The 16% HPW-NH2-SBA-15 is highly efficient in the reaction with a DCPD conversion of 100% and (2) selectivity up to 97%. It is interesting that this material could be reused six times without any significant loss of activity and selectivity. The good stability can be attributed to the strong interaction between the amino groups on the surface of SBA-15 and HPW anions.
1. Introduction
The dicyclopentadiene di-epoxide (2), which is widely used in various fields, such as insulating materials, adhesives, construction materials and electronic parts, is usually prepared from the oxidation of DCPD using various oxidants. In these reactions, the most commonly used catalysts are phase transfer catalysts,1 which are obtained by complicated processes. The use of toxic chlorocarbon solvents, such as chloroform and 1,2-dichloroethane, leads to serious environmental problems and catalyst deactivation is a limitation of the reaction system.2 Recently, 2 was synthesized in 84% selectivity by the epoxidation of DCPD with hydrogen peroxide, catalysed by quaternary ammonium heteropolyphosphato tungstates.3 It is obvious that this condition gives low selectivity of 2. Other catalysts such as H3PW12O40 (HPW)/nano-SiO2 catalysts with Keggin structures were prepared by impregnation and were used in the epoxidation of DCPD with H2O2.4 Under optimal conditions, conversion of DCPD, selectivity for 2 and efficiency of hydrogen peroxide utilization are all higher than 95%. However, leaching of HPW species in these catalysts has restricted their further application. Thus, no green and low-cost process has been applied in industry till now. So it is a great challenge to develop highly efficient and stable catalysts for the above reaction to reach the requirements of practical use.Heteropolyacids (HPAs) are early transition metal oxygen anion clusters.5 Among the various HPA structural classes, Keggin-type6 HPAs have been widely used as homogeneous and heterogeneous catalysts for acid–base and oxidation reactions.7,8 Recently, HPA catalysts have been supported on inorganic porous materials including mesoporous molecular sieves (MCM-419 and SBA-1510), carbon gels11 and mesoporous γ-alumina12 by an impregnation method. However, there is obviously detectable leaching of active species from these catalysts. In recent years, another promising approach to obtain HPA catalysts with high surface areas is to take advantage of the overall negative charge of the heteropolyanions. Using this method, HPA was immobilized on polymer materials such as poly-4-vinylpyridine13 and polyaniline14 to obtain molecularly dispersed HPA catalysts. However, the procedure is restricted when inorganic supporting materials are utilized due to the difficulty in forming a positive charge on inorganic supporting materials. Recently, a successful example for the immobilization of an HPA catalyst on an inorganic support has been reported.15 However, no attempt has been made to use aminopropyl-immobilized phosphotungstic acid on SBA-15 as a catalyst for the preparation of 2 from DCPD.
In this work, tungstic acid was firstly used as an efficient homogeneous catalyst for the target reaction. However, the difficulties of separating and recovering the catalyst from the product mixture during the homogeneous process made it impractical for a large-scale process. Then, aminopropyl-immobilized phosphotungstic acid on SBA-15 was used as the catalyst for the green manufacture of diepoxide from DCPD by aqueous H2O2. The characterisation of HPW-NH2-SBA-15 was extensively performed by various physicochemical techniques.
2. Experimental section
2.1 Catalyst preparation
SBA-15 was prepared following a procedure similar to that reported by Zhao et al.16 The surface modification of the as-obtained SBA-15 was achieved by reacting a silanol group onto SBA-15 with 3-aminopropyltriethoxysilane (APTES, Aldrich) under a nitrogen atmosphere. In detail, 2.3 mmol of APTES was slowly added to a dry toluene solution containing 1 g of SBA-15 with constant stirring at room temperature. The solid products were filtered and dried at 100 °C for 2 h to yield NH2-SBA-15. The immobilization of HPW onto the NH2-SBA-15 support was carried out as follows: 1 g of NH2-SBA-15 was added to an aqueous solution containing HPW (0.36 g) with vigorous stirring at 60 °C, and the resulting solution was maintained at 60 °C for 24 h. The as-obtained solid product was filtered, washed and then dried overnight at 80 °C to yield the 16% HPW-NH2-SBA-15 samples.The impregnation-method-derived HPW/SBA-15 catalyst was synthesized as follows: 0.36 g of HPW was dissolved in distilled water. Then, 1 g of SBA-15 was added into the stirred solution at 60 °C. After stirring for 8 h, the excess water was completely evaporated at the same temperature under depressed pressure and the catalyst (denoted as 16% HPW/SBA-15) was finally obtained after the solid material was dried at 80 °C in air for 3 h.
2.2 Characterizations
The specific surface area, pore volumes and the average pore size of the samples were measured and calculated according to the BET method on Micromeritics TriStar 3000 equipment with liquid nitrogen at −196 °C. Transmission electron micrographs (TEMs) were obtained on a JOEL JEM 2010 scanning transmission electron microscope. The wide-angle X-ray powder diffraction (WAXS) patterns were recorded on a Bruker D8 Advance diffractometer with Cu-Kα radiation, operated at 40 mA and 40 kV. The 31P CP-MAS NMR spectra of the solid catalysts were collected using a Brucker DXR 400 spectrometer. The laser Raman experiments were performed using a Jobin Yvon Dilor Labram I Raman spectrometer equipped with a holographic notch filter, a CCD detector and a He-Ne laser radiating at 632.8 nm. The tungsten content was determined by the inductively coupled plasma (ICP) method (IRIS Intrepid, Thermo Elemental Company) after solubilization of the samples in HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl solutions.
HCl solutions.
2.3 Catalytic reaction: COD oxidation
The activity test was performed at 40 or 60 °C for 20 h with magnetic stirring in a closed 25 mL regular glass reactor using 50% aqueous H2O2 as the oxygen-donor and t-BuOH as the solvent. In a typical experiment, 11.2 mmol of DCPD, 0.1 mol of t-BuOH and 0.46 mmol of the WO3·H2O or 0.2 g of the HPW-NH2-SBA-15 material (16%) were introduced into the regular glass reactor at 60 °C with vigorous stirring. The reaction was started by adding 22.4 mmol of 50 wt% aqueous H2O2 into the mixture and was kept stirring for certain time. The quantitative analyses of the reaction products were performed using gas chromatography (GC) and the identification of different products in the reaction mixture was determined by gas chromatography-mass spectrometry (GC-MS) on HP 6890GC/5973 mass spectrometer.3. Results and discussion
3.1. Tungstic acid catalysis
As we know, homogeneous catalysts are the most common and classical catalysts in aqueous oxidation reactions, because they can be dissolved in the reaction system and show very good activity. Herein, we report a green procedure for the production of 2 by catalytic oxidation of DCPD with aqueous H2O2. The reaction is carried out under mild conditions and presents good selectivity. t-BuOH is chosen as the solvent because the t-BuOH-H2O2 system is stable and safe and has been employed in many oxidation reactions.17 Tungstic acid is an inexpensive catalyst, as compared with other organometallic or noble metal catalysts, and aqueous hydrogen peroxide is a relatively safe and non-polluting oxidant. Thus, providing the solvent and catalyst are recycled, this process can be classed as a green process.According to the GC-MS analysis, the products of DCPD oxidation under these conditions, as shown in Scheme 1, consist of the target product (2) with small amounts of by-products, including 1 and 3. All the products are derivatives from the epoxidation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. It is surprising to find that there are no cleavage products from one or two C
C bonds. It is surprising to find that there are no cleavage products from one or two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.
C bonds.
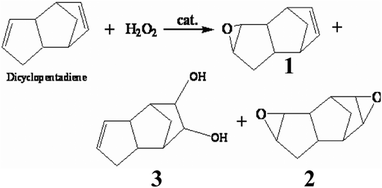 | ||
| Scheme 1 The oxidation products of DCPD by H2O2. | ||
Fig. 1 shows typical plots of DCPD consumption and products formation vs. time under the mild reaction conditions. It is illustrated that a nearly complete conversion of DCPD was achieved after 2 h. It is interesting to find that the yield of 1, which has the highest yield in the early stages, declined with increasing time. For the target product 2, its yield increased initially and then decreased with prolonged reaction time, while the yield of 3 increased with reaction time.
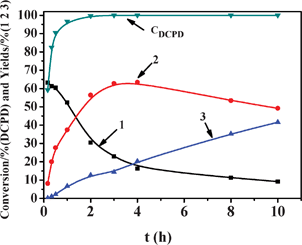 | ||
| Fig. 1 Conversion of DCPD and yields of 1, 2 and 3 over the catalyst. (Reaction conditions: reaction temperature 50 °C, WO3·H2O 0.46 mmol, H2O2 22.4 mmol, DCPD 11.2 mmol, t-BuOH 0.1 mol). | ||
Table 1 shows the selective oxidation of DCPD over various molar ratios of DCPD to H2O2. It can be seen that the conversion of DCPD rose with the increase of molar ratio of H2O2 to DCPD, as did the selectivity for 2. Because H2O2 is the oxygen donor, which plays an important role in the rate of the reaction and the selectivity of products. The complete consumption of DCPD could not be achieved when the ratio is lower than 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At the molar ratio of 2
1. At the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the selectivity for the target product reaches 63.0%. At the molar ratio of 2.5
1, the selectivity for the target product reaches 63.0%. At the molar ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the selectivity for the target product reached 63.2%. Considering the good catalytic performance and H2O2 utility, the 2
1, the selectivity for the target product reached 63.2%. Considering the good catalytic performance and H2O2 utility, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the H2O2 to DCPD was chosen as the optimal value in the following activity test.
1 ratio of the H2O2 to DCPD was chosen as the optimal value in the following activity test.
DCPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2 |
Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| DCPD | H2O2 | 1 | 2 | 3 | |
| a Reaction conditions: reaction temperature 40 °C, H2O2 22.4 mmol, WO3·H2O 0.46 mmol, reaction time 6 h, t-BuOH 0.1 mol. | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
99.4 | 97.3 | 38.4 | 54.3 | 7.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 96.9 | 23.7 | 63.0 | 13.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
100 | 88.1 | 20.5 | 63.2 | 16.3 |
As we know, the solvent also plays an important role in the activity as well as the selectivity of the reaction. As shown in Table 2, the best DCPD conversion and the highest selectivity for the target product were observed when the solvent was t-BuOH and ethanol, whereas other solvents resulted in low DCPD conversion. However, the intrinsic nature of the different kinds of solvent is still not clear yet.
| Solvent | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| DCPD | H2O2 | 1 | 2 | 3 | |
| a Reaction conditions: reaction temperature 40 °C, reaction time 4 h, H2O2 22.4 mmol, WO3·H2O 0.46 mmol, DCPD 11.2 mmol. | |||||
| Methanol | 99.3 | 94.3 | 48.9 | 41.9 | 9.2 |
| Ethanol | 100 | 94.3 | 50.5 | 44.7 | 4.8 |
| i-Propanol | 98.8 | 94.9 | 50.6 | 46.6 | 2.8 |
| n-Butanol | 72.4 | 98.1 | 69.7 | 28.3 | 2.0 |
| t-BuOH | 100 | 90.5 | 50.0 | 44.2 | 5.8 |
| Tetrahydrofuran | 30.1 | 24.6 | 88.6 | 9.9 | 1.5 |
| 1,4-Dioxane | 78.9 | 84.0 | 70.6 | 27.3 | 2.1 |
Independent experiments were carried out to study the effect of the molar amount of t-BuOH on the reaction. Table 3 indicates that there is an optimum amount of t-BuOH at which a maximum amount of 2 is formed. If the molar ratio of t-BuOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCPD was in the range of 4.5–13.3, the selectivity for the target product was similar. Further increasing the molar ratio to 17.8 resulted in an obvious decrease in the selectivity. This finding can be explained by the fact that, for the formation of 2, an appropriate concentration of active oxidizing species is required. The concentration of active oxidizing species formed depends on the concentration of hydrogen peroxide. An excess amount of t-BuOH leads to a lower concentration of oxidizing species.
DCPD was in the range of 4.5–13.3, the selectivity for the target product was similar. Further increasing the molar ratio to 17.8 resulted in an obvious decrease in the selectivity. This finding can be explained by the fact that, for the formation of 2, an appropriate concentration of active oxidizing species is required. The concentration of active oxidizing species formed depends on the concentration of hydrogen peroxide. An excess amount of t-BuOH leads to a lower concentration of oxidizing species.
| t- BuOH/mol |
t- BuOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCPD DCPD |
Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| DCPD | H2O2 | 1 | 2 | 3 | ||
| a Reaction conditions: reaction temperature 40 °C, reaction time 6 h, H2O2 22.4 mmol, WO3·H2O 0.46 mmol, DCPD 11.2 mmol. | ||||||
| 0.05 | 4.5 | 100 | 95.2 | 8.8 | 61.8 | 29.4 |
| 0.1 | 8.9 | 100 | 96.9 | 23.7 | 63.0 | 13.3 |
| 0.15 | 13.4 | 100 | 93.9 | 24.7 | 63.1 | 12.2 |
| 0.2 | 17.9 | 98.7 | 88.1 | 50.6 | 45.1 | 4.3 |
Table 4 shows the effect of reaction temperature. The reaction temperature plays an important role in the conversion of DCPD and the yield of 2. The conversion of DCPD increases with increasing temperature until 60 °C and then remains at 100%. However, the yield of 2 initially increases with the temperature until 50 °C, and then decreases abruptly with further increase of the temperature to 60 °C.
| T/°C | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| DCPD | H2O2 | 1 | 2 | 3 | |
| a Reaction conditions: reaction time 2 h, H2O2 22.4 mmol, WO3·H2O 0.46 mmol, DCPD 11.2 mmol. | |||||
| 30 | 78.4 | 82.9 | 71.8 | 27.0 | 1.2 |
| 40 | 97.4 | 90.5 | 50.0 | 44.2 | 5.8 |
| 50 | 99.7 | 94.4 | 30.6 | 56.6 | 12.8 |
| 60 | 100 | 97.3 | 20.7 | 55.6 | 23.6 |
| 70 | 100 | 96.0 | 27.4 | 46.0 | 26.6 |
Though the homogeneous catalyst, H2WO4, is efficient in this reaction, and can be recovered and reused, the post-treatment is too complicated and a high content of tungsten contaminant was found in the final products. These drawbacks make this process very inconvenient. Therefore, the problem diverts to the finding of new catalysts that are easily recovered and reused without any leaching of tungsten species into the reaction mixture.
3.2. HPW-NH2-SBA-15 catalysis
Heterogeneous catalysts are also used in most liquid reactions, especially mesoporous materials with the active sites doped in. Their popularity reflects the findings that these materials have some unique advantages. Their large surface area and unique porous structure makes them available for the metal or the metallic oxides to disperse well on their surface. In addition, these insoluble solids are easily recovered by simple filtration or centrifugation. In the present work, HPW-NH2-SBA-15 materials were successfully synthesized via an immobilized method, and they presented excellent activity and selectivity in the selective oxidation of DCPD to 2 with aqueous H2O2, if compared with the homogeneous tungstic acid catalyst.| Catalysts | W (%) | mmol amine / g silica | mmol HPA / g silica | mol HPA / mol amine |
|---|---|---|---|---|
| a Determined according to the ICP-AES method. | ||||
| 8% HPW-NH2-SBA-15 | 6.9 | 2.2 | 0.031 | 0.0141 |
| 16% HPW-NH2-SBA-15 | 15.5 | 2.2 | 0.070 | 0.0318 |
| 24% HPW-NH2-SBA-15 | 24.5 | 2.2 | 0.110 | 0.0500 |
| 30% HPW-NH2-SBA-15 | 27.9 | 2.2 | 0.125 | 0.0568 |
| 16% HPW/SBA-15 | 16.3 | — | — | — |
N2 adsorption isotherms for different HPW-NH2-SBA-15 samples are also recorded. Irreversible-type IV adsorption isotherms with H1 hysteresis loops, defined by IUPAC, are observed, which show a typical feature of mesoporous materials (Fig. 2). The textural parameters of catalysts with different HPW loadings are listed in Table 6. The surface area of NH2-SBA-15 silica decreased with the incorporation of APTES. HPW-NH2-SBA-15 silica has a much lower surface area than the corresponding NH2-SBA-15 silica, due to the loading of the HPW species. The surface area of HPW-NH2-SBA-15, as well as pore volume, dropped along with increasing HPW loadings.
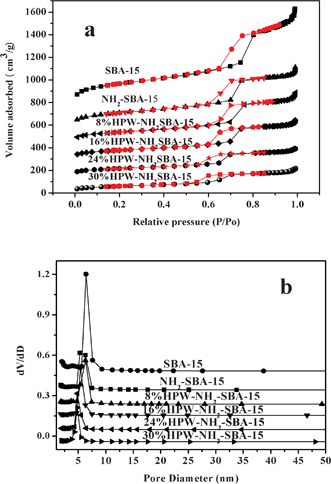 | ||
| Fig. 2 (a) Nitrogen adsorption–desorption isotherms and (b) the corresponding pore size distribution for various samples. | ||
| Material | SBET/m2 g−1 | Vp/cm3 g−1 |
|---|---|---|
| SBA-15 | 760 | 1.37 |
| NH2-SBA-15 | 407 | 0.80 |
| 8% HPW-NH2-SBA-15 | 323 | 0.68 |
| 16% HPW-NH2-SBA-15 | 284 | 0.54 |
| 24% HPW-NH2-SBA-15 | 239 | 0.38 |
| 30% HPW-NH2-SBA-15 | 214 | 0.35 |
| 16% HPW/SBA-15 | 463 | 0.84 |
Fig. 3 shows TEM images of 16% HPW-NH2-SBA-15, revealing the characteristic structural feature of the SBA-15 materials.16 This result clearly indicates that the samples keep the unique pore structure of the parent support very well. Fig. 4 shows the XRD patterns of bulk HPW and HPW-NH2-SBA-15 samples. The bulk HPW shows the typical patterns of Keggin anion structure. On the other hand, HPW-NH2-SBA-15 samples show no typical peaks related to Keggin structure except the peaks attributed to the amorphous silica. This finding indicates that the HPW species on the HPW-NH2-SBA-15 samples were not in a crystalline state but in a highly mono-dispersed state. It is believed that those heteropolyanions were strongly immobilized on NH2-SBA-15 as charge compensating components. Thus, it is reasonable that the HPW species are finely and molecularly dispersed on the surface of NH2-SBA-15 via a chemical bonding interaction.
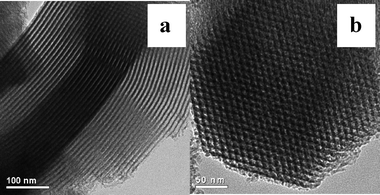 | ||
| Fig. 3 TEM images of 16% HPW-NH2-SBA-15 (a: perpendicular to the channel direction of SBA-15, b: parallel to the channel direction of SBA-15). | ||
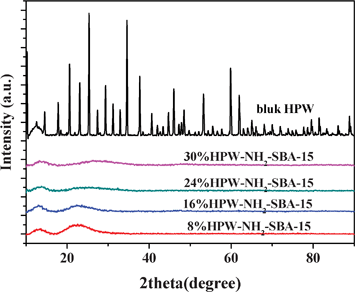 | ||
| Fig. 4 XRD patterns of pure HPW and HPW-NH2-SBA-15 samples. | ||
The HPW-NH2-SBA-15 and HPW/SBA-15 catalysts were all characterized by Raman spectroscopy, as shown in Fig. 5. The bulk HPW and 16% HPW/SBA-15 samples show Raman bands at 216, 233, 996 and 1009 cm−1. Those peaks can easily be assigned to the Keggin structure of the PW12O403− anion. The very strong band at 1009 cm−1 is characteristic of highly condensed polymeric phosphotungstate with Keggin structure, being attributed to υs(WOt) (Ot = terminal oxygen). The strong band at 996 cm−1 is assigned to υas(WOt) and the band at 216 cm−1 can be attributed to υs(W–Oμ) (Oμ = oxygen in bridge or μ-oxo).18 In Fig. 5, for HPW-NH2-SBA-15 catalysts, broad Raman bands at 940 cm−1 appear, which can be ascribed to highly dispersed, isolated HPW species. This finding suggests that the HPW species are finely and molecularly dispersed on the surface of NH2-SBA-15. In addition, the Raman spectrum of 16% HPW/SBA-15 is similar to that of the bulk HPW, indicating that the HPW species over 16% HPW/SBA-15 show some characteristics of bulk HPW. Thus, the HPW species are not well dispersed on the SBA-15 support for the impregnated 16% HPW/SBA-15 catalyst.
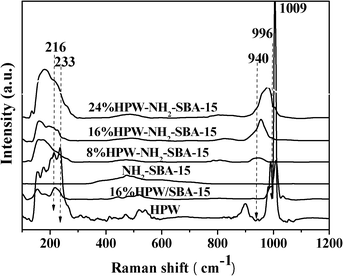 | ||
| Fig. 5 Raman spectroscopy of the HPW-NH2-SBA-15 catalysts and HPW/SBA-15 samples. | ||
31P MAS-NMR is a useful tool in detecting the local environments of HPWs, since the chemical shift of the phosphorous atom depends not only on its local environment within the metal cluster, but also on factors such as associated water molecules, metal ions, solid supports and thermal treatments.19,20Fig. 6 shows the 31P MAS-NMR spectra of HPW-NH2-SBA-15 and 16% HPW/SBA-15 samples. The 16% HPW/SBA-15 catalyst exhibited a sharp, intense peak at −15.3 ppm, typical of Keggin structures.19 The sharp peak indicates the presence of P in a highly uniform environment in the hydrated structure of polyoxometalate (POM). After immobilization, the 31P NMR peaks were found to shift in the upfield direction. The upfield shift reflects the interaction of [PW12O40]3− with the cationic mesoporous silica support. The shift in peak position and decrease in the peak width can also be associated with the loss of water molecules during POM loading.21 It is interesting to note that the upfield shift was more exaggerated for 8% HPW-NH2-SBA-15 than that for 30% HPW-NH2-SBA-15. This difference in behavior for the two samples can be attributed to a higher HPW loading in the 30% HPW-NH2-SBA-15 sample, in which excessive HPW particles show some condensed polymeric HPW species character, while the HPW species of 8% HPW-NH2-SBA-15 are presented as totally isolated species. In addition, the NMR pattern of 16% HPW/SBA-15 is similar to that of the bulk HPW, indicating that the HPW species of 16% HPW/SBA-15 show some character of bulk HPW. Thus, the HPW species are not well dispersed on the SBA-15 support for the impregnated 16% HPW/SBA-15 catalyst. This finding indicates that there is weak interaction between HPW and the SBA-15 silica support for the traditional impregnation method derived catalysts.
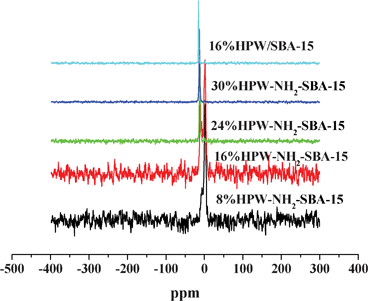 | ||
| Fig. 6 The 31P MAS-NMR spectra of various samples. | ||
| Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|
| Catalyst | DCPD | H2O2 | 2 | Other products |
| a Reaction conditions: reaction temperature 60 °C, reaction time 20 h, DCPD 11.2 mmol, H2O2 22.4 mmol, cat. 0.4 g, t-BuOH 0.1 mol. | ||||
| 8% HPW-NH2-SBA-15 | 88.8 | 52.7 | 38.9 | 61.1 |
| 16% HPW-NH2-SBA -15 | 100 | 98.9 | 97.0 | 3.0 |
| 24% HPW-NH2-SBA-15 | 100 | 97.8 | 63.8 | 36.1 |
| 30% HPW-NH2-SBA-15 | 100 | 97.0 | 71.8 | 28.2 |
| 16% HPW/SBA-15 | 100 | 95.9 | 56.9 | 43.1 |
The reusability and regeneration of 16% HPW-NH2-SBA-15 and 16% HPW/SBA-15 are also listed in Table 8. The DCPD conversion and the selectivity for 2 over the immobilized method derived 16%HPW-NH2-SBA-15 catalyst decrease slowly but remain above 100% and 96.9% after the 6th cycle, while these two values over the 16%HPW/SBA-15 catalyst are only 46.2% and 36.8% after the 3rd cycle of the reaction. This finding clearly indicates that the immobilized method derived 16%HPW-NH2-SBA-15 catalyst shows much better stability and can be reused at least 6 times, while the impregnation method derived one can only be used once. That is why the HPW materials are very difficult to apply in commercial plants. To investigate the stability of the active tungsten species in the 16% HPW-NH2-SBA-15 catalyst, the reaction mixture and the tungsten remaining in the catalyst were also determined by ICP analysis after five reaction cycles. No detectable leaching of tungsten species or obvious loss of tungsten in the 16% HPW-NH2-SBA-15 catalyst could be observed, however, the leaching of tungsten species from 16% HPW/SBA-15 is 1000 ppm in one reaction cycle. Therefore, it can be concluded that the interaction between the active HPW species and the SBA-15 support from the immobilized method is much stronger than that of HPW/SBA-15 prepared by the traditional impregnation method. To the best of our knowledge, the as-prepared 16% HPW-NH2-SBA-15 material is the first example of HPW based catalysts used in the selective oxidation of DCPD without any leaching of tungsten species and loss of catalytic activity with aqueous H2O2 as the oxidant.
| Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|
| Catalyst | Entry | DCPD | H2O2 | 2 | Other products |
| a Reaction conditions: reaction temperature 60 °C, reaction time 20 h, DCPD 11.2 mmol, H2O2 22.4 mmol, cat. 0.4 g, t-BuOH 0.1 mol. | |||||
| 16% HPW-NH2-SBA-15 | 1 | 100 | 98.9 | 97.0 | 3.0 |
| 2 | 100 | 97.5 | 96.8 | 3.2 | |
| 3 | 100 | 96.3 | 96.9 | 3.1 | |
| 4 | 100 | 98.1 | 97.0 | 3.0 | |
| 5 | 100 | 97.2 | 96.7 | 3.3 | |
| 6 | 100 | 96.9 | 96.9 | 3.1 | |
| 16% HPW/SBA-15 | 1 | 100 | 95.9 | 56.9 | 43.1 |
| 2 | 97.0 | 95.3 | 45.5 | 54.5 | |
| 3 | 46.2 | 35.6 | 36.8 | 63.2 | |
In addition, another experiment was carried out to test whether this novel 16% HPW-NH2-SBA-15 catalyst is actually a heterogeneous one (see Fig. 7). The reaction over 16% HPW-NH2-SBA-15 was carried out for 2 h, at which point the catalyst was removed through simple filtration and the reaction solution was stirred for another 22 h. No detectable increase of DCPD conversion in the next 22 h of reaction was observed, indicating that the 16% HPW-NH2-SBA-15 catalyst is actually a heterogeneous one. It was also interesting to find that the addition of new DCPD to the solution, which had been left stirring for 24 h after the heterogeneous catalyst was removed, resulted in zero conversion of DCPD in another 24 h. In view of the excellent activity, selectivity and stability of the HPW-NH2-SBA-15 material in the selective oxidation of DCPD with aqueous H2O2, further studies on the utilization of this material in other green organic oxidations including epoxidation and oxidative cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds with aqueous H2O2 are under way.
C bonds with aqueous H2O2 are under way.
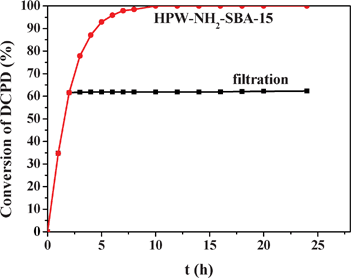 | ||
| Fig. 7 The conversion of DCPD with time over the 16%HPW-NH2-SBA-15 and the filtration. | ||
4. Conclusion
Oxidation of DCPD to 2 was carried out on two kinds of catalyst material. The tungsten acid catalyst gave a high yield of 63% of 2. XRD and Raman spectroscopy indicated that the HPW species on HPW-NH2-SBA-15 samples are finely dispersed on the surface of NH2-SBA-15 via chemical bonding interactions. The 31P MAS-NMR studies uncovered strong interactions between tungsten heteropolyacids and the immobilized silica. The 16% HPW-NH2-SBA-15 catalyst was highly efficient for the production of 2 with a DCPD conversion of 100% and (2) selectivity up to 97%. It is worth mentioning that this material could be reused six times without any significant loss of activity and selectivity. The 16% HPW-NH2-SBA-15 catalyst exhibited better stability for the selective oxidation of DCPD than the 16% HPW/SBA-15 catalyst. The good stability can be attributed to the strong interaction between the amino groups on the surface of SBA-15 and HPW anions.Acknowledgements
This work was financially supported by the Major State Basic Resource Development Program (Grant No. 2012CB224804), NNSFC (Project 20973042, 21173052), the Research Fund for the Doctoral Program of Higher Education (20090071110011) and the Natural Science Foundation of Shanghai Science and Technology Committee (08DZ2270500), Research Fund for the innovation Program of Shanghai University (A.10040711003).References
- Y. S. Wang, Y. D. Zhang and Z. X. Wang, Chin. J. Appl. Chem., 2010, 27, 1021 CAS.
- C. Qin, F. Hu, B. Liu and Y. S. Jiao, J. East China Univer. Sci. Technol., 2004, 30, 669 CAS.
- X. C. Li, X. M. Wu and A. B. Tang, Fine Chem. Intermed., 2010, 40, 54 CAS.
- L. Li, G. Li, J. Peng and L. Zhang, Petrochem. Tech., 2006, 35, 1130 CAS.
- M.T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, New York, 1983 Search PubMed.
- J. F. Keggin, Nature, 1933, 131, 908 CrossRef CAS.
- I. V. Kozhevnikov, Catal. Rev. Sci. Eng., 1995, 37, 311 CAS.
- C. L. Hill and C. M. Prosser-McCartha, Coord. Chem. Rev., 1995, 143, 407 CrossRef CAS.
- W. Chu, X. Yang, Y. Shan, X. Ye and Y. Wu, Catal. Lett., 1996, 42, 201 CrossRef CAS.
- N. Y. He, C. S. Woo, H. G. Kim and H. I. Lee, Appl. Catal., A, 2005, 281, 167 CrossRef CAS.
- K. Nowinska, R. Formaniak and W. A. Waclaw, Appl. Catal., A, 2003, 256, 115 CrossRef CAS.
- P. Kim, H. Kim, J. Yi and I. K. Song, Stud. Surf. Sci. Catal., 2006, 159, 265 CrossRef CAS.
- K. Nomiya, H. Murasaki and M. Miwa, Polyhedron, 1986, 5, 1031 CrossRef CAS.
- M. Hasik, W. Turek, E. Stochmal, M. Łapkowski and A. Pron, J. Catal., 1994, 147, 544 CrossRef CAS.
- H. Kim, P. Kim, K. Y. Lee, S. H. Yeom, J. Yi and I. K. Song, Catal. Today, 2006, 111, 361 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- X. L. Yang, W. L. Dai, H. Chen, Y. Cao, H. X. Li, H. Y. He and K. N. Fan, J. Catal., 2005, 229, 259 CrossRef CAS.
- C. R'Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Inorg. Chem., 1983, 22, 207–216 CrossRef.
- C. J. Dillon, J. H. Holles, R. J. Davis, J. A. Labinger and M. E. Davis, J. Catal., 2003, 218, 54 CrossRef CAS.
- S. Uchida, K. Inumaru and M. Misono, J. Phys. Chem. B, 2000, 104, 8108 CrossRef CAS.
- S. Damyanova, J. L. G. Fierro, I. Sobrados and J. Sanz, Langmuir, 1999, 15, 469 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
