DOI:
10.1039/C2RA20777J
(Paper)
RSC Adv., 2012,
2, 7081-7086
New organic dyes containing tert-Butyl-capped N-Arylcarbazole moiety for Dye-sensitized solar cells
Received
26th April 2012
, Accepted 29th May 2012
First published on 30th May 2012
Abstract
Two new organic dyes with tert-butyl-capped N-arylcarbazole as a donor, cyanoacrylic acid as an acceptor and a bithiophene unit as a π-linker (DH-11 and DH-12) have been synthesized and characterized for dye-sensitized solar cells (DSSCs). It is found that the introduction of tert-butyl-capped N-arylcarbazole as an electron donor can efficiently suppress the intermolecular aggregation and improve the photovoltaic performances. The DSSC devices based on the dyes show relatively high power conversion efficiency of 3.67 and 3.75% for DH-11 and DH-12, respectively, which reaches over 65% of the reference dye N719-based cell fabricated and measured under the same conditions. This infers that the tert-butyl-capped N-arylcarbazole unit is a promising electron-donor that can be employed to design metal-free sensitizers with a new structural skeleton.
1. Introduction
Since the first report by O'Regan and Grätzel in 1991,1 dye-sensitized solar cells (DSSCs) have aroused great interest in both scientists and engineers due to their high efficiencies and low cost which make it a powerful competitor to conventional silicon-based photovoltaic devices.2
As one of the most crucial factor that affects the performance of DSSCs, suitable organic and organometallic dyes have attracted considerable research attention.3–5 Thanks to researchers' remarkable efforts, a high power conversion efficiency (η) of over 11% has been attained for some DSSCs of Ru(II)-based sensitizers under the condition of air mass (AM) 1.5 illumination.6 However, compared with organic metal-free dyes, the cost and inconvenience of purifying Ru(II)-based sensitizers greatly limits their development. Therefore, metal-free organic dyes have become promising candidates for DSSCs due to their high molar extinction coefficient, low cost, and excellent flexibility in terms of molecular modification and tailoring.7
In the last decade, various organic functional groups have been arranged and combined to generate D-π-A structure for organic sensitizers. Among them, the arylamine group, thiophene or oligothiophene unit, and cyanoacrylic acid moieties are the most commonly employed subunits as the electron donor, π- linker, and electron acceptor/anchoring group, respectively.8–12 A common strategy to design the new structure of an organic sensitizer with more efficient harvesting of solar energy is to introduce a longer π-conjugated backbone that can yield much red-shifted absorption spectra.13,14 However, extension of the coplanar π-electron structure can result in self-aggregation of the dye molecules on the surface of TiO2 film and lead to inefficient electron injection into the conduction band of TiO2.15,16 The non-planar structure of the triphenylamine group can slightly suppress this unfavorable process.17 Moreover, the introduction of some bulky structural features in the organic dyes is one of most effective approaches to further prevent intermolecular aggregation.
Considering the non-planar structure of the triphenylamine group, direct connection of two interfacing phenyl groups will form a planar carbazole group that makes another N-substituted phenyl group almost perpendicular to the carbazole group via a sp3-hybridized nitrogen atom. Thus, it has been widely used in organic optoelectronics for its distinct optoelectronic properties.18–20 Since the steric rigidity of the N-arylcarbazole group can efficiently suppress intermolecular interactions and diminish aggregation, the introduction of N-arylcarbazole group as an electron-donor will be very favorable for improving the performance of DSSCs. Along this line we design and synthesize two new metal-free organic dyes (DH-11 and DH-12, as shown in Fig. 1) for DSSCs, in which the N-arylcarbazole moiety acts as an electron-donor to link the cyanoacrylic acid moieties as an electron acceptor/anchoring group via a bithiophene bridge. In order to further suppress the intermolecular aggregation, improve the solubility and enhance the light-resistant ability of the sensitizer,9 the tert-butyl group is chosen to attach to the donor group for the first time. Here, we investigate and present their photophysical, electrochemical properties and photovoltaic performances.
 |
| | Fig. 1 Molecular structure of DH-11 and DH-12 dyes. | |
2. Experimental
2.1 Equipment
1H-NMR and 13C-NMR spectra were measured with a Varian MERCURY-VX300 in CDCl3 or DMSO-d6 with TMS as the internal reference. UV-vis absorption spectrometry was performed on a Shimadzu UV-3600 spectrophotometer. The electrochemical behaviors of DH dyes were investigated using cyclic voltammetry (CV) on a CHI600A electrochemical work station, the cyclic voltammograms of DH dyes were measured in a solution of 0.1M n-Bu4NPF6 in dichloromethane. A three-electrode cell containing a Pt-coil working electrode, a Pt wire counter electrode and an Ag/AgCl reference electrode were employed. The ferrocene/ferricenium (Fc/Fc+) redox couple was used as an internal reference. Prior to the electrochemical measurements, all solutions were degassed with high-purity argon. The elemental analysis was carried out on a CARLOERBA-1106 microelemental analyzer. Mass spectra were recorded with a VJ-ZAB-3F-Mass spectrometer or a Bruker 320-MS triple quadrupole mass spectrometer.
2.2 Materials
The catalyst of Pd(PPh3)4 was synthesized in our own lab, and anhydrous THF and toluene used in the Schlenk system were purified by refluxing with a Na-K alloy. Compound 1,212,216,227,23 were prepared according to the corresponding literatures. All the other reagents and solvents were used as commercially purchased without further purification.
2.3 Preparation and fabrication of DSSCs
The preparation of the photoelectrode was performed by adopting the doctor-blade technique on a conducting glass (FTO, 15–20 Ω sq−1), which has been rinsed with distilled water and fully soaked in isopropanol for 3h before use to increase its hydrophilicity. The TiO2 paste containing TiO2 with a diameter 13 nm (Ti-Nanoxide D, Solaronix) was spread on FTO glass. Four pieces of FTO glass with adhesive tape placed as a spacer were put in parallel when spreading the paste in order to obtain films with the same thickness. The films were dried under mild conditions and then annealed at 500 °C for 1 h. Finally, the dye-sensitized electrodes were prepared by immersing the obtained films in a mixed solution of dyes (ethanol/THF for DH-11 and DH-12) and N71924 (ethanol, 3 × 10−4 mol L−1 of all) overnight. The dye-sensitized electrode was assembled in a classic sandwich-type cell, that is, the Platinum counter electrode was attached on the dye-sensitized photoanode, after the injection of the electrolyte solution, which consists of 0.5 M LiI, 0.05 M I2, and 0.1 M 4-tert-butylpyridine in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propylene carbonate into the interspaces between the photoanode and the counter electrode, the photoelectrochemical property of DSSC was measured. For the characteristic photocurrent-voltage (J-V) measurements, the DSSC was illuminated by light with energy of a 100 mW cm−2 (AM 1.5) from a 300 W solar simulator (Newport, 91160). A computer-controlled Keithley 2400 source meter was employed to collect the J-V curves. The incident photon-to-current conversion efficiency (IPCE) was measured as a function of wavelength from 300 to 800 nm by using a Model QE/IPCE system (PV Measurements Inc.).
propylene carbonate into the interspaces between the photoanode and the counter electrode, the photoelectrochemical property of DSSC was measured. For the characteristic photocurrent-voltage (J-V) measurements, the DSSC was illuminated by light with energy of a 100 mW cm−2 (AM 1.5) from a 300 W solar simulator (Newport, 91160). A computer-controlled Keithley 2400 source meter was employed to collect the J-V curves. The incident photon-to-current conversion efficiency (IPCE) was measured as a function of wavelength from 300 to 800 nm by using a Model QE/IPCE system (PV Measurements Inc.).
2.4 Synthesis
3-Tert-butyl-9-(4-tert-butylphenyl)-9H-carbazole (3).
A Schlenk tube containing 3-tert-butyl-9H-carbazole (2) (4.46 g, 20.0 mmol), 1-bromo-4-tert-butylbenzene (5.33 g, 25.0 mmol), Pd(OAc)2 (674 mg, 1.0 mmol), t-Bu3P (404 mg, 2 mmol), t-BuONa (5.76 mg, 60 mmol) and anhydrous toluene (80 mL) equipped with a magnetic stirrer was heated at 120 °C for 36 h under argon atmosphere. The reaction mixture was then filtered and washed with hexane. The filtrate was evaporated to dryness. The final product (3, white solid) was purified by silica gel column chromatography with petroleum ether. Yield, 5.39 g (76%). 1H NMR (300 MHz, CDCl3): δ 8.14 (s, 1H), 7.60 (d, 2H, J = 8.4 Hz), 7.47–7.45 (m, 4H), 7.39–7.36 (m, 3H), 7.26 (br, 1H), 1.45 (s, 9H), 1.42 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 150.1, 142.7, 141.2, 139.1, 135.1, 129.7, 126.7, 126.3, 125.5, 123.7, 123.5, 120.0, 119.5, 116.2, 109.8, 109.4, 34.7, 31.9, 31.4. MS(ESI): m/z = 356.7. C26H29N (Mw = 355.52): calcd. C, 87.84; H, 8.22; N, 3.94. found C, 87.53; H, 8.21; N, 3.88.
3-Bromo-6-tert-butyl-9-(4-tert-butylphenyl)-9H-carbazole (4).
To a 3-tert-butyl-9-(4-tert-butylphenyl)-9H-carbazole (3) (3.55 g, 10 mmol) in DMF (40 mL), a solution of N-bromosuccinimide (1.78 g, 10 mmol) in DMF (10 mL) was added dropwise. After stirring overnight at room temperature, the resulting brown solution was poured into ice water (100 mL) and extracted with dichloromethane (60 mL × 3). The organic phase was washed with water and brine and dried over anhydrous Na2SO4. After filtration, the solvent was removed and the residue was purified by column chromatography on silica gel using petroleum ether as the eluent to obtain the target compounds as a white solid. Yield, 3.87 g (89%). 1H NMR (300 MHz, CDCl3): δ 8.25 (s, 1H), 8.01(s, 1H), 7.59 (d, 2H, J = 8.7 Hz), 7.50 (d, 2H, J = 8.7 Hz), 7.43 (d, 2H, J = 8.7 Hz), 7.35 (d, 1H, J = 8.4 Hz), 7.27 (d, 1H, J = 8.7 Hz), 1.44 (s, 9H), 1.42 (s, 9H). 13C NMR (75MHz, CDCl3): δ 144.8, 138.4, 137.2, 132.8, 130.4, 129.4, 129.2, 128.1, 127.0, 126.5, 126.1, 125.6, 122.8, 115.6, 112.4, 104.0, 34.9, 32.0, 31.7. MS (ESI): m/z = 434.7. C26H28BrN (Mw = 434.41), calcd.: C, 71.89; H, 6.50; N, 3.22. Found: C, 71.63; H, 6.93; N, 3.05.
3-Tert-butyl-9-(4-tert-butylphenyl)-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (5).
A solution of 3-bromo-6-tert-butyl-9-(4-tert-butylphenyl)-9H-carbazole (4) (3.48 g, 8 mmol) in anhydrous THF (60 mL) was treated with n-BuLi (3.5 mL, 2.4 M in hexane, 8.4 mmol) under argon at −78 °C. After stirring for 50 min, 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.56 g, 8.4 mmol) was added. The resulting solution was stirred for 1 h at −78 °C, and allowed to warm to room temperature. After 12 h, the reaction was quenched with methanol, and the organic layer was extracted with ether (40 mL × 3), then washed with water and brine, dried over anhydrous Na2SO4. After filtration, the solvent was removed with a rotary evaporator and the residue was purified by column chromatography on silica gel using CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give out the product as a pale yellow solid. Yield, 3.47 g (7.2 mmol, 90%). 1H NMR (300 MHz, CDCl3): δ 8.76 (s, 1H), 8.31 (s, 1H), 7.93 (d, 1H, J = 7.2 Hz), 7.66–7.64 (m, 2H), 7.54–7.41 (m, 5H), 1.53 (s, 9H), 1.52 (s, 9H), 1.48 (s, 12H). 13C NMR (75 MHz, CDCl3): δ150.6, 143.6, 143.51, 143.50, 139.6, 135.2, 132.4, 127.9, 127.0, 126.6, 124.0, 123.6, 123.5, 117.0, 109.7, 109.5, 83.9, 35.1, 35.0, 32.3, 31.8, 25.2. MS (ESI): m/z = 482.6. C32H40BNO2 (Mw = 481.48): calcd. C, 79.83; H, 8.37; N, 2.91; found C, 80.18; H, 8.10; N, 2.79.
1) as eluent to give out the product as a pale yellow solid. Yield, 3.47 g (7.2 mmol, 90%). 1H NMR (300 MHz, CDCl3): δ 8.76 (s, 1H), 8.31 (s, 1H), 7.93 (d, 1H, J = 7.2 Hz), 7.66–7.64 (m, 2H), 7.54–7.41 (m, 5H), 1.53 (s, 9H), 1.52 (s, 9H), 1.48 (s, 12H). 13C NMR (75 MHz, CDCl3): δ150.6, 143.6, 143.51, 143.50, 139.6, 135.2, 132.4, 127.9, 127.0, 126.6, 124.0, 123.6, 123.5, 117.0, 109.7, 109.5, 83.9, 35.1, 35.0, 32.3, 31.8, 25.2. MS (ESI): m/z = 482.6. C32H40BNO2 (Mw = 481.48): calcd. C, 79.83; H, 8.37; N, 2.91; found C, 80.18; H, 8.10; N, 2.79.
5′-(6-Tert-butyl-9-(4-tert-butylphenyl)-9H-carbazol-3-yl)-2,2′-bithiophene-5-carbaldehyde (8).
In a Schlenk tube, 3-tert-butyl-9-(4-tert-butylphenyl)-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (5) (722 mg, 1.5 mmol), 5′-bromo-2,2′-bithiophene-5-carbaldehyde (6) (382 mg, 1.4 mmol), Pd(PPh3)4 (93 mg, 0.8 mmol) and K2CO3 (829 mg, 6 mmol) were dissolved in a degassed mixed solvent of THF/H2O (20 mL/4 mL). The mixture was heated at 65 °C under an argon atmosphere for 2d. After reaction, the organic phase was separated, dried over anhydrous Na2SO4 and filtered. The filtrate was evaporated to dryness. The final product (9, orange-red solid) was purified by silica gel column chromatography with CH2Cl2/petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. Yield, 712 mg (1.3 mmol, 92%). 1H NMR (300 MHz, CDCl3): δ 9.86 (s, 1H), 8.34 (s, 1H), 8.18 (s, 1H), 7.69 (d, 1H, J = 3.9 Hz), 7.62–7.59 (m, 3H), 7.50–7.43 (m, 4H), 7.39–7.36 (m, 2H), 7.33 (d, 1H, J = 3.9 Hz), 7.28 (d, 1H, J = 3.9 Hz), 1.48 (s, 9H), 1.45 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 182.6, 150.6, 148.0, 147.9, 143.6, 141.3, 141.1, 139.8, 137.8, 134.9, 133.9, 127.5, 127.0, 126.4, 125.5, 124.6, 124.2, 124.0, 123.7, 123.3, 122.9, 117.7, 116.7, 110.5, 110.0, 35.0, 32.2, 31.6. MS (ESI): m/z = 548.1. C35H33NOS2 (Mw = 547.77): calcd. C, 76.74; H, 6.07; N, 2.56.; found C, 76.33; H, 6.05; N, 2.79.
1) as eluent. Yield, 712 mg (1.3 mmol, 92%). 1H NMR (300 MHz, CDCl3): δ 9.86 (s, 1H), 8.34 (s, 1H), 8.18 (s, 1H), 7.69 (d, 1H, J = 3.9 Hz), 7.62–7.59 (m, 3H), 7.50–7.43 (m, 4H), 7.39–7.36 (m, 2H), 7.33 (d, 1H, J = 3.9 Hz), 7.28 (d, 1H, J = 3.9 Hz), 1.48 (s, 9H), 1.45 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 182.6, 150.6, 148.0, 147.9, 143.6, 141.3, 141.1, 139.8, 137.8, 134.9, 133.9, 127.5, 127.0, 126.4, 125.5, 124.6, 124.2, 124.0, 123.7, 123.3, 122.9, 117.7, 116.7, 110.5, 110.0, 35.0, 32.2, 31.6. MS (ESI): m/z = 548.1. C35H33NOS2 (Mw = 547.77): calcd. C, 76.74; H, 6.07; N, 2.56.; found C, 76.33; H, 6.05; N, 2.79.
5′-(6-tert-butyl-9-(4-tert-butylphenyl)-9H-carbazol-3-yl)-3,3′-dihexyl-2,2′-bithiophene-5-carbaldehyde (9).
The synthetic procedure of compound 10 was similar to compound 9, instead of 5′-bromo-2,2′-bithiophene-5-carbaldehyde (7), 5′-bromo-3,3′-dihexyl -2,2′-bithiophene-5-carbaldehyde (8, 441 mg, 1 mmol) was used with compound 6 (505 mg, 1.05 mmol) for the reaction, gave out the aldehyde 10 as an orange sticky oil. Yield, 630 mg (0.88 mmol, 88%). 1H NMR (300 MHz, CDCl3): δ 9.87 (s, 1H), 8.35 (s, 1H), 8.17 (s, 1H), 7.70 (s, 1H), 7.64–7.59 (m, 3H), 7.49–7.46 (m, 2H), 7.42–7.36 (m, 2H), 7.27–7.25 (m, 2H), 2.69–2.57 (m, 4H), 1.63–1.54(m, 4H), 1.47 (s, 9H), 1.43 (s, 9H), 1.28–1.26 (m, 12H), 0.87–0.83 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 182.8, 150.6, 146.8, 144.4, 143.6, 143.5, 142.6, 141.2, 140.6, 140.0, 137.9, 137.7, 135.2, 129.2, 127.0, 126.6, 126.1, 125.6, 124.5, 124.2, 123.2, 117.2, 116.7, 110.5, 110.0, 34.9, 32.3, 31.7, 31.1, 31.0, 30.8, 30.0, 29.6, 29.4, 29.2, 29.0, 22.9, 14.4. MS (ESI): m/z = 715.2.
(E)-3-(5′-(6-tert-butyl-9-(4-tert-butylphenyl)-9H–carbazol-3-yl)-2,2′-bithiophen-5-yl)-2-cyanoacrylic acid (DH-11).
A mixture of the aldehyde 8 (438 mg, 0.8 mmol), cyanoacetic acid (68 mg, 8 mmol), ammonium acetate (77 mg, 1 mmol), and glacial acetic acid (8 mL) was heated at 135 °C for 24 h. After cooling to room temperature, the resulting precipitate was filtered off and washed with glacial acetic acid to give the pure target dye (DH-11) as a brown solid. Yield, 296 mg (0.38 mmol, 76%) 1H NMR (300 MHz, d6-DMSO): δ 8.75 (s, 1H), 8.50 (s, 1H), 8.44 (s, 1H), 8.00 (d, 1H, J = 3.6 Hz), 7.77 (d, 1H, J = 8.7 Hz), 7.71–7.68 (m, 4H), 7.62 (d, 1H, J = 3.9 Hz), 7.57–7.52 (m, 3H), 7.38 (d, 1H, J = 8.4 Hz), 7.32 (d, 1H, J = 8.7 Hz), 1.43 (s, 9H), 1.40 (s, 9H). 13C NMR (75 MHz, d6-DMSO): δ 164.4, 150.6, 147.7, 147.0, 146.9, 143.7, 141.7, 142.4, 140.9, 139.5, 134.8, 134.1, 133.4, 129.0, 127.5, 126.4, 125.4, 125.1, 125.0, 124.6, 124.4, 123.1, 118.4, 117.9, 117.3, 110.7, 110.0, 98.0, 35.2, 35.1, 32.4, 31.8. MS (ESI): m/z = 613.9. C38H34N2O2S2 (Mw = 614.82): calcd. C, 74.23; H, 5.57; N, 4.56.; found C, 74.13; H, 5.45; N, 4.82.
(E)-3-(5′-(6-tert-butyl-9-(4-tert-butylphenyl)-9H-carbazol-3-yl)-3,3′-dihexyl-2,2′-bithiophen-5-yl)-2-cyanoacrylic acid (DH-12).
The synthetic procedure of DH-12 is similar to that of DH-11, in which the corresponding aldehyde 9 (573 mg, 0.8 mmol) was used to produce the dye DH-12 as a crimson solid. Yield, 431 mg (0.55 mmol, 69%). 1H NMR (300 MHz, d6-DMSO): δ 8.67 (s, 1H), 8.47 (s, 1H), 8.38 (s, 1H), 7.98 (s, 1H), 7.70 (s, 1H), 7.68 (d, 2H, J = 8.4 Hz), 7.60 (s, 1H), 7.55–7.50 (m, 3H), 7.37 (d, 1H, J = 8.4 Hz), 7.31 (d, 1H, J = 9.0 Hz), 2.64–2.55 (m, 4H), 1.63–1.57 (m, 4H), 1.42 (s, 9H), 1.39 (s, 9H), 1.22 (m, 12H), 0.82–0.80 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 168.7, 150.7, 148.1, 147.3, 144.9, 144.2, 143.6, 143.2, 141.3, 140.2, 139.9, 135.0, 134.7, 127.0, 126.8, 126.5, 125.8, 125.0, 124.5, 124.3, 124.2, 123.1, 117.67, 116.8, 115.8, 110.5, 110.0, 96.9, 35.0, 32.2, 31.9, 31.8, 31.7, 31.1, 30.8, 29.8, 29.4, 29.3, 29.1, 22.8, 14.4. MS (ESI): m/z = 783.7. C50H58N2O2S2 (Mw = 783.14): calcd. C, 76.68; H, 7.46; N, 3.58.; found C, 76.63; H, 7.15; N, 3.83.
3. Results and discussion
3.1 Synthesis and characterization
The synthetic route of the DH dyes is depicted in Scheme 1. The key intermediate 3-tert-butyl-9H-carbazole (2) was obtained via a photostimulated reaction in two steps with high yield.21 Buchwald–Hartwig C–N coupling was adopted to synthesize the tert-butyl-capped N-arylcarbazole (3). After bromination, the monobrominated N-arylcarbazole (4) was treated with n-BuLi to eliminate the halogen atom at −78 °C, and 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane was added to yield the corresponding 2,3-dimethyl-2,3-butanediol boronate (5). Then 5 reacts with α-monobromobithiophene aldehyde (6 and 7) via Suzuki coupling to produce the corresponding compounds (8) and (9), respectively. Finally, the cyanoacetic acid moiety was connected to 8 or 9via Knoevenagel condensation to give the target dyes in high yields.
 |
| | Scheme 1 Synthetic routes for dyes DH-11 and DH-12 | |
3.2 Photophysical and electrochemical properties
The UV–vis absorption spectra of DH dyes in solution and absorbed on TiO2 films are presented in Fig. 2 and the corresponding data are summarized in Table 1. As can be seen, both of the dyes exhibit absorption bands at two distinct spectrum regions. One is at about 340 nm, corresponding to the π–π* electron transition of the conjugated skeleton. The absorption band above 350 nm is assigned to an intramolecular charge transfer (ICT) between the electron donor (tripheylamine) and the electron acceptor (cyanoacetic acid anchoring moieties). By comparison, it is found that the absorption maximum of ICT for DH-11 is clearly more red-shifted than that of DH-12, since the steric effect of two hexyl groups at ortho β positions of bithiophene unit destroys the coplanar skeleton of DH-12 and also weakens the ICT effect. However, compared with UV-vis absorption in solution, the dyes adsorbed on the surface of the TiO2 film show a dramatically red-shifted absorption band. In particular, the absorption intensity from 300 to 550 nm is significantly enhanced owing to the increased delocalization of the π* orbital of the conjugated framework caused by the interaction between the carboxylate group and the Ti4+ ions which directly decreases the energy of the π* level. In addition, both the dyes show high extinction coefficients, as shown in Table 1, indicating that their light-harvesting performance may be very efficient.
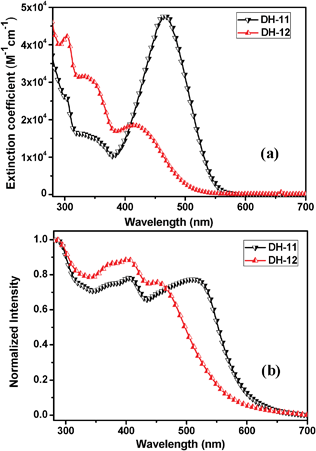 |
| | Fig. 2 UV-vis spectra of DH dyes in a solution of THF (a), normalized absorption profiles of dyes in the TiO2 film (b). | |
Table 1 Optical and electrochemical properties of DH dyes
| Sample |
λ
max
(nm)/ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) c (10 4 M−1cm−1) c (10 4 M−1cm−1) |
λ
max
(nm) |
E
ox
(V) |
E
opt
(V) |
HOMO f(eV) |
LUMO g (eV) |
|
Absorption maxima in solution of THF.
Absorption maxima adsorbed on TiO2 film.
The molar extinction coefficient at λmax in solution of THF.
Oxidation potentials with reference to the ferrocene which was used as an internal standard.
Calculated with the formula Eopt = 1240/λonset.
Calculated with the formula EHOMO = −{Eox + [4.8 – E(Fc/Fc+)]} eV.
Calculated with the formula ELUMO = (EHOMO + Eopt) eV.
|
|
DH-11
|
465/4.79 |
405, 510 |
0.91 |
2.07 |
−5.36 |
−3.29 |
|
DH-12
|
335/3.15, 417/1.85 |
403, 451 |
0.93 |
2.12 |
−5.38 |
−3.26 |
The redox behavior of DH dyes was studied by cyclic voltammetry (in Fig. 3) for the purpose of investigating the electron transfer ability from the excited dye molecules to the conductive band of TiO2.
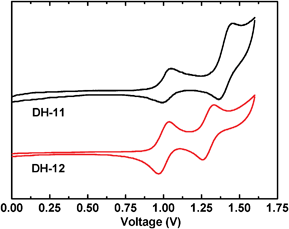 |
| | Fig. 3 Cyclic voltammogram of DH dyes. | |
As can be seen in Fig. 3 and Table 1, the onset reductive potentials of DH dyes are around 0.92 V which give LUMO energies of DH dyes of around −3.28 eV, which are higher than the TiO2 conduction band (−3.9 eV),25,26 as shown in Fig. 4. This indicates that the electron injection process is energetically favorable compared with the conduction band edge energy level of the TiO2 electrode (0.5 V vs. NHE).27 The onset oxidation potential around 0.92 eV can be attributed to the oxidation of the N-arylcarbazole group and their HOMO energies of around −5.37 eV are lower than the I−/I3− potential, suggesting that there is enough driving force for the dye's regeneration.
 |
| | Fig. 4 Energy level diagrams of DH dyes from electrochemical data.24,25 | |
3.3 Theoretical approaches
The frontier molecular orbital of the dyes at the B3LYP/6-31G level are shown in Fig. 5, the surfaces are generated with an isovalue of 0.02. As can be seen, the electron density is uniformly distributed along the N-arylcarbazole unit at the HOMO state. At the LUMO level, the excited electrons are shifted to the π-electron system of the anchoring groups (bithiophene-substituted cyanoacrylic acid unit), which can be attributed to the ICT along the π-conjugated skeleton. Therefore, it can be expected that the excited electron will be effectively injected into the conduction band of TiO2 through the carboxyl anchoring group as well as the adjacent electron-withdrawing cyano group. Moreover, the results of calculation indicate that the introduction of the 3,3′-dihexyl-2,2′-bithiophene group in DH-12 is more beneficial to the spatial separation of HOMO and LUMO energy levels by comparison of DH-11. The twisted nonplanar skeleton28–30 and alkyl-substituted structure31 for DH-12 can suppress the charge recombination and enhance the open-circuit voltage more effectively, which has been confirmed by the following photovoltaic studies.
 |
| | Fig. 5 The frontier HOMO and LUMO orbitals of DH dyes. | |
3.4 Photovoltaic performances
The photovoltaic properties of the solar cells based on DH dyes were measured under standard AM 1.5 G irradiation. The photocurrent density–voltage (J-V) curves and the incident photon to current conversion efficiency (IPCE) spectra of DSSCs based on DH-11 and DH-12 are shown in Fig. 6, and the results are summarized in Table 2.
 |
| | Fig. 6 J-V curves and IPCE spectra of DH dyes. | |
Table 2 Photovoltaic performances of DSSCs based on DH dyes and N719
| Sample |
J
sc (mA cm−2) |
V
oc (V) |
FF |
η (%) |
|
DH-11
|
10.02 |
0.57 |
0.64 |
3.62 |
|
DH-12
|
9.44 |
0.66 |
0.63 |
3.75 |
| N719 |
12.27 |
0.72 |
0.63 |
5.58 |
As can be seen, both DH-11 and DH-12 show favorable photovoltaic responses and exhibit relatively high power conversion efficiencies. The overall power conversion efficiency (η) of 3.62% for DH-11 and 3.75% for DH-12 reaches 64.9% and 67.2% of a N719-based device, respectively. It should be noted that both of the DH dyes have almost same fill factor (FF) value. By comparison, the short circuit current density (Jsc) of 10.02 mA cm−2 for the cell based on DH-11 is higher than that of the DH-12-based cell (Jsc = 9.44 mA cm−2), which can be mainly attributed to its highly coplanar structure that causes more efficient light-harvesting, stronger ICT absorption and higher molar extinction coefficient. Compared with DH-11, the introduction of ortho-long-chain alkyl-substituted bithiophene structure in DH-12 partially destroys the coplanarity of the conjugated skeleton that is unfavorable for its absorption with broadened UV-vis spectra and high extinction coefficient. However, the twisted nonplanar structure results in the charge separation of the dye molecule and suppresses the charge recombination that leads to the enhancement of an open circuit voltage (Voc). Thus, the Voc of 0.66 V for DH-12 is much higher than that of DH-11 (Voc = 0.57 V), which is very similar to the result of another kind of organic dye containing twisted nonplanar or coplanar bithiophene unit recently reported by Wang et al.28. In addition, the IPCE spectra is in agreement with the result of Jsc,32 so the DH-11-based cell shows considerable response from 350 to 650 nm compared to the reference dye N719 based cell, while the DH-12-based cell only gives a moderate IPCE response in line with the trend in the molar extinction coefficient of these dyes (Table 1 and Fig. 2).
4. Conclusions
A new kind of metal-free organic dye composed of the tert-butyl-capped N-arylcarbazole moiety as an electron-donor and the cyanoacrylic acid moiety as an electron-acceptor linked by a bithiophene bridge has been designed and synthesized. It is found that the existence of the tert-butyl-capped N-arylcarbazole unit suppresses the intermolecular aggregation, and the unsubstituted bithiophene unit as a conjugation bridge enhances the molecular rigidity and co-planarity which is favorable for light-harvesting and improvement of the Jsc of the cell. However, the steric effect of the hexyl group at ortho β-positions of bithiophene group destroys co-planarity of the molecular skeleton and improves the effective spatial charge separation that enhances the Voc of the cell. The relative high energy conversion efficiency of the DSSC devices based on the DH dyes suggests that the tert-butyl-capped N-arylcarbazole unit can act as an effective electron-donor to construct a new kind of metal-free organic sensitizer for DSSCs. The further optimization of photovoltaic performances, modification of structure and exploration of the relationship between structure and photovoltaic properties is still in progress.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (Nos. 20972122 and 51173138), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20100141110010) for financial support.
References
- B. O'Reagen and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- L. M. Gonçalves, V. d. Z Bermudez, H. A Ribeiro and A. Magalhães Mendes, Energy Environ. Sci., 2008, 1, 655 Search PubMed.
- M. K. Nazeeruddin, C. Klein, P. Liska and M. Grätzel, Coord. Chem. Rev., 2005, 249, 1460 CrossRef CAS.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338 CrossRef CAS.
- Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 18, 2891 CrossRef.
- C. Y. Chen, M. K. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-le, J. D. Decoppet, J. H. Tsai, C. Grätzel, C. G. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103 CrossRef CAS.
- A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS.
- H. Qin, S. Wenger, M. Xu, F. Gao, X. Jing, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 9202 CrossRef CAS.
- J. H. Yum, D. P. Hagberg, S. J. Moon, K. M. Karlsson, T. Marinado, L. C. Sun, A. Hagfeldt, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2009, 48, 1576 CrossRef CAS.
- G. L. Zhang, H. Bala, Y. M. Cheng, D. Shi, X. J. Lv, Q. J. Yu and P. Wang, Chem. Commun., 2009, 16, 2198 RSC.
- H. Im, S. Kim, C. Park, S. H. Jang, C. J. Kim, K. Kim, N. G. Park and C. Kim, Chem. Commun., 2010, 46, 1335 RSC.
- W. D. Zeng, Y. M. Cao, Y. Bai, Y. H. Wang, Y. S. Shi, M. Zhang, F. F. Wang, C. Y. Pan and P. Wang, Chem. Mater., 2010, 22, 1915 CrossRef CAS.
- C. Kim, H. Choi, S. Kim, C. Baik, K. Song, M. S. Kang, S. O. Kang and J. Ko, J. Org. Chem., 2008, 73, 7072 CrossRef CAS.
- H. Y. Yang, Y. S. Yen, Y. C. Hsu, H. H. Chou and J. T. Lin, Org. Lett., 2010, 12, 16 CrossRef CAS.
- Z. Ning, Q. Zhang, W. Wu, H. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3791 CrossRef CAS.
- Z. Ning, Q. Zhang, H. Pei, J. Luan, C. Lu, Y. Cui and H. Tian, J. Phys. Chem. C, 2009, 113, 10307 CAS.
- G. Li, K. J. Jiang, Y. F. Li, S. L. Li and L. M. Yang, J. Phys. Chem. C, 2008, 112, 11591 CAS.
- K. Brunner, A. van Dijken, H. Borner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, J. Am. Chem. Soc., 2004, 126, 6035 CrossRef CAS.
- D. Kim, J. K. Lee, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 1913 CrossRef CAS.
- Y. D. Zhang, C. Zuniga, S. J. Kim, D. K. Cai, S. Barlow, S. Salman, V. Coropceanu, J-L. Breédas, B. Kippelen and S. Marder, Chem. Mater., 2011, 23, 4002 CrossRef CAS.
- M. E. Budeén, V. A. Vaillard, S. E. Martin and R. A. Rossi, J. Org. Chem., 2009, 74, 4490 CrossRef.
- L. J. Song, P. Amaladass, S. H. Wen, K. K. Pasunooti, A. Li, Y. L. Yu, X. Wang, W. Q. Deng and X. W. Liu, New J. Chem., 2011, 35, 127–136 RSC.
- N. Hayashi, T. Nishihara, T. Matsukihira, H. Nakashima, K. Miyabayashi, M. Miyake and H. Higuchi, Bull. Chem. Soc. Jpn., 2007, 80, 371 CrossRef CAS.
- M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulos, V. Shklover, C. H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298 CrossRef CAS.
- F. Lenzmann, J. Krueger, S. Burnside, K. Brooks, M. Grätzel, D. Gal, S. Ruhle and D. Cahen, J. Phys. Chem. B, 2001, 105, 6347 CrossRef CAS.
- D. Kumaresan, R. P. Thummel, T. Bura, G. Ulrich and R. Ziessel, Chem.–Eur. J., 2009, 15, 6335 CrossRef CAS.
- Z. S. Wang, Y. Cui, Y. Dan-Oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2008, 112, 17011 CAS.
- M. Xu, M. Zhang, M. Pastore, R. Li, F. De Angelis and P. Wang, Chem. Sci., 2012, 3, 976 RSC.
- M. Velusamy, K. R. J. Thomas, J. T. Lin, Y. C. Hsu and K. C. Ho, Org. Lett., 2005, 7, 1899 CrossRef CAS.
- T. N. Duan, K. Fan, Y. Fu, C. Zhong, X. G. Chen, T. Y. Peng and J. G. Qin, Dyes Pigm., 2012, 94, 28 CrossRef CAS.
- M. F. Xu, R. Z. Li, N. Pootrakulchote, D. Shi, J. Guo, Z. H. Yi, S. M. Zakeeruddin, M. Grätzel and P. Wang, J. Phys. Chem. C, 2008, 112, 19770 CAS.
- M. Grätzel, Inorg. Chem., 2005, 44, 6841 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propylene carbonate into the interspaces between the photoanode and the counter electrode, the photoelectrochemical property of DSSC was measured. For the characteristic photocurrent-voltage (J-V) measurements, the DSSC was illuminated by light with energy of a 100 mW cm−2 (AM 1.5) from a 300 W solar simulator (Newport, 91160). A computer-controlled Keithley 2400 source meter was employed to collect the J-V curves. The incident photon-to-current conversion efficiency (IPCE) was measured as a function of wavelength from 300 to 800 nm by using a Model QE/IPCE system (PV Measurements Inc.).
propylene carbonate into the interspaces between the photoanode and the counter electrode, the photoelectrochemical property of DSSC was measured. For the characteristic photocurrent-voltage (J-V) measurements, the DSSC was illuminated by light with energy of a 100 mW cm−2 (AM 1.5) from a 300 W solar simulator (Newport, 91160). A computer-controlled Keithley 2400 source meter was employed to collect the J-V curves. The incident photon-to-current conversion efficiency (IPCE) was measured as a function of wavelength from 300 to 800 nm by using a Model QE/IPCE system (PV Measurements Inc.).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give out the product as a pale yellow solid. Yield, 3.47 g (7.2 mmol, 90%). 1H NMR (300 MHz, CDCl3): δ 8.76 (s, 1H), 8.31 (s, 1H), 7.93 (d, 1H, J = 7.2 Hz), 7.66–7.64 (m, 2H), 7.54–7.41 (m, 5H), 1.53 (s, 9H), 1.52 (s, 9H), 1.48 (s, 12H). 13C NMR (75 MHz, CDCl3): δ150.6, 143.6, 143.51, 143.50, 139.6, 135.2, 132.4, 127.9, 127.0, 126.6, 124.0, 123.6, 123.5, 117.0, 109.7, 109.5, 83.9, 35.1, 35.0, 32.3, 31.8, 25.2. MS (ESI): m/z = 482.6. C32H40BNO2 (Mw = 481.48): calcd. C, 79.83; H, 8.37; N, 2.91; found C, 80.18; H, 8.10; N, 2.79.
1) as eluent to give out the product as a pale yellow solid. Yield, 3.47 g (7.2 mmol, 90%). 1H NMR (300 MHz, CDCl3): δ 8.76 (s, 1H), 8.31 (s, 1H), 7.93 (d, 1H, J = 7.2 Hz), 7.66–7.64 (m, 2H), 7.54–7.41 (m, 5H), 1.53 (s, 9H), 1.52 (s, 9H), 1.48 (s, 12H). 13C NMR (75 MHz, CDCl3): δ150.6, 143.6, 143.51, 143.50, 139.6, 135.2, 132.4, 127.9, 127.0, 126.6, 124.0, 123.6, 123.5, 117.0, 109.7, 109.5, 83.9, 35.1, 35.0, 32.3, 31.8, 25.2. MS (ESI): m/z = 482.6. C32H40BNO2 (Mw = 481.48): calcd. C, 79.83; H, 8.37; N, 2.91; found C, 80.18; H, 8.10; N, 2.79.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. Yield, 712 mg (1.3 mmol, 92%). 1H NMR (300 MHz, CDCl3): δ 9.86 (s, 1H), 8.34 (s, 1H), 8.18 (s, 1H), 7.69 (d, 1H, J = 3.9 Hz), 7.62–7.59 (m, 3H), 7.50–7.43 (m, 4H), 7.39–7.36 (m, 2H), 7.33 (d, 1H, J = 3.9 Hz), 7.28 (d, 1H, J = 3.9 Hz), 1.48 (s, 9H), 1.45 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 182.6, 150.6, 148.0, 147.9, 143.6, 141.3, 141.1, 139.8, 137.8, 134.9, 133.9, 127.5, 127.0, 126.4, 125.5, 124.6, 124.2, 124.0, 123.7, 123.3, 122.9, 117.7, 116.7, 110.5, 110.0, 35.0, 32.2, 31.6. MS (ESI): m/z = 548.1. C35H33NOS2 (Mw = 547.77): calcd. C, 76.74; H, 6.07; N, 2.56.; found C, 76.33; H, 6.05; N, 2.79.
1) as eluent. Yield, 712 mg (1.3 mmol, 92%). 1H NMR (300 MHz, CDCl3): δ 9.86 (s, 1H), 8.34 (s, 1H), 8.18 (s, 1H), 7.69 (d, 1H, J = 3.9 Hz), 7.62–7.59 (m, 3H), 7.50–7.43 (m, 4H), 7.39–7.36 (m, 2H), 7.33 (d, 1H, J = 3.9 Hz), 7.28 (d, 1H, J = 3.9 Hz), 1.48 (s, 9H), 1.45 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 182.6, 150.6, 148.0, 147.9, 143.6, 141.3, 141.1, 139.8, 137.8, 134.9, 133.9, 127.5, 127.0, 126.4, 125.5, 124.6, 124.2, 124.0, 123.7, 123.3, 122.9, 117.7, 116.7, 110.5, 110.0, 35.0, 32.2, 31.6. MS (ESI): m/z = 548.1. C35H33NOS2 (Mw = 547.77): calcd. C, 76.74; H, 6.07; N, 2.56.; found C, 76.33; H, 6.05; N, 2.79.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) c (10 4 M−1cm−1)
c (10 4 M−1cm−1)



