Metal-free pinnick-type oxidative amidation of aldehydes†‡
Kien Soon
Goh
a and
Choon-Hong
Tan
*b
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543
bDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371. E-mail: choonhong@ntu.edu.sg
First published on 28th May 2012
Abstract
A metal-free Pinnick-type oxidative amidation of aldehyde has been developed. Sodium chlorite was found to be a useful oxidant in coupling an aldehyde and amine to form an amide bond. A large variety of substrates are suitable and many functional groups are tolerated due to the mild nature of this amidation methodology. Optically active substrates were coupled smoothly with good yield and without racemization. As inexpensive reagents were used, it is cost effective even on a larger scale, indicating its potential for practical application.
The ubiquitous presence of amide linkages in a multitude of important compounds, makes amide bond formation one of the most important transformations in organic chemistry.1a,1b For the same reason, the prevalence of the amide moiety, it sometimes gives the wrong impression that there are no remaining synthetic challenges.1c,1d Amides are typically synthesized through the conversion of carboxylic acids to acyl chlorides and subsequent coupling with an amine. Alternatively, carboxylic acid and the amine can be coupled directly by using a stoichiometric amount of activating agents like carbodiimides.1e Increasing concern for environmental sustainability and economic effectiveness have led to a concerted effort by the community to find new reagents and approaches for preparing the amide bond.1c,1d
Oxidative amidation using aldehydes2a–m has received much attention in recent years. The direct formation of amide from aldehydes using readily available starting materials avoids the isolation of intermediates, reducing cost and wastage.2a The oxidative amidation of aldehydes with amines can be catalyzed with transition metals such as Rh,2b Ru,2c Pd2d and lanthanides.2e Subsequently, a Cu-catalyzed version using tert-butyl hydroperoxide (TBHP) as an oxidant and silver iodate was developed.2f Metal-free oxidative amidation methods of aromatic aldehydes with secondary amine have been shown to be possible with only TBHP.2g Another version using potassium iodide as the catalyst and TBHP as the oxidant was developed and had a wider scope.2h However, the coupling between an aliphatic aldehyde and a primary amine remains challenging. No metal-free version of the oxidative amidation of alcohols has been reported.3a–e
Several other contemporary amidation methods have also emerged.4–10 An umpolung approach to form amides from α-bromonitroalkanes and amines was developed with N-iodosuccinimide and K2CO3.4 The α-bromonitroalkane acts as the nucleophile and N-iodoamine acts as an electrophile, reversing the traditional role of the acyl donor and amine. In another method, boronic acid is used as the coupling agent, which generates an active ester from carboxylic acid.5 It subsequently couples to an amine in a waste-free catalytic manner. N-Heterocyclic carbene (NHC), acting as a catalyst, was also found to generate an active carboxylate, which is converted to an amide with a variety of amines.6 Alkynes have been used to couple to amine to form amides; under a basic-oxidative condition, both aromatic and aliphatic alkynes reacted with amines in the presence of a manganese-porphyrin7a or copper(II)/TEMPO catalyst.7b Finally, thioacid was shown to couple with azide to form amides under catalyst-free conditions.8
The oxidation of aldehyde to carboxylic acid using sodium chlorite in the presence of an HClO scavenger like sulphamic acid and resorcinol was first reported by Lindgren et al.9 The potential of this mild method of oxidation was also anticipated and reported by Kraus et al. for its ability to keep sensitive functional groups intact in complex systems.10 Subsequently, the oxidation of α,β-unsaturated aldehydes or sterically hindered groups was reported by Pinnick et al. using 2,3-dimethylbut-2-ene as the HClO scavenger.11 Imine was also found to be oxidized to amide using Pinnick oxidation with a limited substrate scope.12
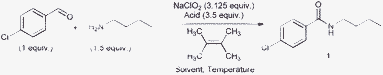
In this paper, we report a one-pot reaction using Pinnink oxidation to couple an aldehyde and amine to give an amide. We investigated the amination between 4-chlorobenzaldehyde and n-butylamine with 3.125 equiv. of NaClO2 and 3.5 equiv. of acid. Several weak acids were screened (Table 1, entries 1–4). Acetic acid gave a 46% yield of the amide with the remaining aldehyde oxidizing to carboxylic acid (entry 1). It was found that NaH2PO4 was able to give the highest yield of amide with minimal oxidation of aldehyde (entry 4).
| Entry | Acid | T/°C | Alkene (equiv.) | Solvent | Yield (%)b |
|---|---|---|---|---|---|
| a Reactions were performed using 4-chlorobenzaldehyde (0.10 mmol) and n-butylamine (0.15 mmol) in 0.5 ml of solvent and allowed to react overnight. b Isolated yield | |||||
| 1 | AcOH | 40 | - | MeCN | 46 |
| 2 | NaHSO4 | 40 | - | MeCN | 42 |
| 3 | NH4Cl | 40 | - | MeCN | 51 |
| 4 | NaH2PO4 | 40 | - | MeCN | 57 |
| 5 | NaH2PO4 | 40 | 1 | MeCN | 62 |
| 6 | NaH2PO4 | 40 | 5 | MeCN | 81 |
| 7 | NaH2PO4 | 40 | 5 | EA | 88 |
| 8 | NaH2PO4 | 40 | 5 | 1,4-Dioxane | 35 |
| 9 | NaH2PO4 | 40 | 5 | tBuOH | 81 |
| 10 | NaH2PO4 | 40 | 5 | Toluene | 96 |
| 11 | NaH2PO4 | 25 | 5 | Toluene | 91 |
| 12 | NaH2PO4 | 40 | 4 | Toluene | 85 |
It was proposed that the aldehyde and amine first undergo a condensation reaction to form an imine (Scheme 1). The imine nitrogen was next protonated by the acid, and the chlorite ion (ClO2−) attacks the imine carbon that subsequently oxidized to an amide. It was also proposed that 2,3-dimethylbut-2-ene can effectively act as a scavenger for the hypochlorite ion.12 We found that 5 equiv. of 2,3-dimethylbut-2-ene can effectively improve the yield of amide (entry 6). Several solvents were tested (Table 1, entries 7–10) and toluene was found to give the highest yield of 96%. We found that the reaction can be carried out at room temperature (entry 11), but the yield decreased slightly to 91%. It is possible to lower the amount of 2,3-dimethylbut-2-ene to 4 equiv. (entry 12), but a slight decrease in yield was observed.
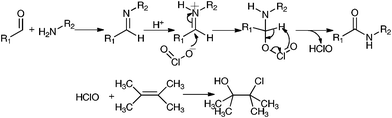 | ||
| Scheme 1 Proposed reaction mechanism. | ||
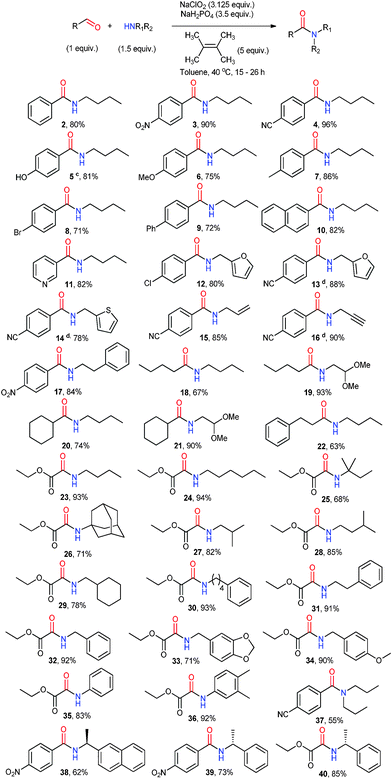
With our optimized conditions, we explored the substrate scope for this reaction (Scheme 2). A large variety of substrates was used as coupling partners to illustrate the general applicability of this method of amidation. In general, the oxidative amidation of aldehyde proceeded well for substituted benzaldehydes and primary amines. Benzaldehyde coupled to n-butylamine affords amide 2 in good yield. Substituted benzaldehydes with strong electron withdrawing substituents like nitro- and nitrile- gave amides 3–9 in excellent yields. Pyridyl- and naphthyl-aldehydes proceeded smoothly with good yields to give the corresponding amides 10 and 11. Amines with furan and thiophene moieties also coupled smoothly with aldehyde, giving amides 12–14 with good yields. To test whether unsaturated bonds could be tolerated during the amide formation, amines with alkene and alkyne functional groups were used as the coupling partners to 4-cyanobenzaldehyde. Amides 15 and 16 were obtained with excellent yields. The oxidative amidation between aliphatic aldehydes and a primary amine is the most difficult to achieve for most oxidative amidation methods reported so far. This methodology was able to couple these partners with no difficulty, giving amides 18–22 in good yields. It was also observed that the acetal protecting group remains intact throughout this reaction to give a high yield of amides 19 and 21. This shows the mild nature of the reaction and its suitability for substrates with weak protecting groups. Ethyl glycoxylate was also used as a coupling partner and gave high yields of amides 23–36. It was shown that amines with bulky groups gave slightly lower yields of 68% and 71% of amides 25 and 26, respectively. Aniline was also found to be an excellent coupling partner, giving amides 35 and 36 with excellent yields. Secondary amine can also couple to 4-cyanobenzaldehyde, giving amide 37 with moderate yield. Finally, we were able to show that chiral substrates can be coupled using this methodology to give amides 38–40 with no racemization observed.
To show the potential for the practical applicability of this methodology, we carried out a large scale reaction coupling ethyl glycoxylate and n-butylamine, which was able to afford excellent yield (Scheme 3).
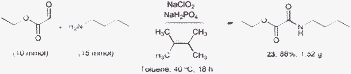 | ||
| Scheme 3 Gram scale amidation experiment. | ||
In summary, we have developed a metal-free oxidative amidation method for aldehyde using Pinnick oxidation. This method has great potential for practical application since a large variety of aldehydes and amines, both optically active and inactive ones, were suitable for the reaction. In addition, the reaction procedure is simple and can be easily carried out without having to synthesize any catalysts. Furthermore, the reagents used are inexpensive and easily available in most common laboratories, thus scalability for this reaction is not an issue. We have successfully performed a gram scale reaction to illustrate this. Further investigation into our proposed mechanism is still in progress.
References
- (a) B. L. Bray, Nat. Rev. Drug Discovery, 2003, 2, 587–593 CrossRef CAS; (b) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243–2266 CrossRef CAS; (c) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS; (d) D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC; (e) F. Albericio, Curr. Opin. Chem. Biol., 2004, 8, 211–221 CrossRef CAS.
- Selected examples of oxidative amidation of aldehydes: (a) K. Ekoue-Kovi and C. Wolf, Chem.–Eur. J., 2008, 14, 6302–6315 CrossRef CAS; (b) A. Tillack, I. Rudloff and M. Beller, Eur. J. Org. Chem., 2001, 523–528 CrossRef CAS; (c) J. W. W. Chang and P. W. H. Chan, Angew. Chem., Int. Ed., 2008, 47, 1138–1140 CrossRef CAS; (d) Y. Suto, N. Yamagiwa and Y. Torisawa, Tetrahedron Lett., 2008, 49, 5732–5735 CrossRef CAS; (e) J. Li, F. Xu, Y. Zhang and Q. Shen, J. Org. Chem., 2009, 74, 2575–2577 CrossRef CAS; (f) W.-J. Yoo and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 13064–13065 CrossRef CAS; (g) K. Ekoue-Kovi and C. Wolf, Org. Lett., 2007, 9, 3429–3432 CrossRef CAS; (h) K. R. Reddy, C. U. Maheswari, M. Venkateshwar and M. L. Kantam, Eur. J. Org. Chem., 2008, 3619–3622 CrossRef CAS; (i) S. Seo and T. J. Marks, Org. Lett., 2008, 10, 317–319 CrossRef CAS; (j) L. Wang, H. Fu, Y. Jiang and Y. Zhao, Chem.–Eur. J., 2008, 14, 10722–10726 CrossRef CAS; (k) J. Gao and G.-W. Wang, J. Org. Chem., 2008, 73, 2955–2958 CrossRef CAS; (l) K. Ishihara and T. Yano, Org. Lett., 2004, 6, 1983–1986 CrossRef CAS; (m) D. Bonne, M. Dekhane and J. Zhu, J. Am. Chem. Soc., 2005, 127, 6926–6927 CrossRef CAS.
- Selected examples of oxidative amidation of alcohols: (a) C. Gunanathan, Y. Ben-David and D. Milstein, Science, 2007, 317, 790–792 CrossRef CAS; (b) J.-F. Soule, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 18550–18553 CrossRef CAS; (c) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Angew. Chem., 2011, 123, 1–6 CrossRef; (d) T. Zweifel, J. V. Naubron and H. Grutzmacher, Angew. Chem., Int. Ed., 2009, 48, 559–563 CrossRef CAS; (e) L. U. Nordstrøm, H. Vogt and R. Madsen, J. Am. Chem. Soc., 2008, 130, 17672–17673 CrossRef.
- B. Shen, D. M. Makley and J. N. Johnston, Nature, 2010, 465, 1027–1033 CrossRef CAS.
- Selected examples of boronic acid-catalyzed amination: (a) T. Maki, K. Ishihara and H. Yamamoto, Org. Lett., 2005, 7, 5043–5046 CrossRef CAS; (b) T. Maki, K. Ishihara and H. Yamamoto, Tetrahedron Lett., 2007, 63, 8645–8657 CAS; (c) R. M. Al-Zoubi, O. Marion and D. G. Hall, Angew. Chem., Int. Ed., 2008, 47, 2876–2879 CrossRef CAS; (d) I. Georgiou, G. Ilyashenko and A. Whiting, Acc. Chem. Res., 2009, 42, 756–768 CrossRef CAS.
- (a) J. W. Bode and S. S. Sohn, J. Am. Chem. Soc., 2007, 129, 13798–13799 CrossRef CAS; (b) P.-C. Chiang, Y. Kim and J. W. Bode, Chem. Commun., 2009, 4566–4568 RSC; (c) H. U. Vora and T. Rovis, J. Am. Chem. Soc., 2007, 129, 13796–13797 CrossRef CAS.
- (a) W.-K. Chan, C.-M. Ho, M.-K. Wong and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 14796–14797 CrossRef CAS; (b) C. Zhang and N. Jiao, J. Am. Chem. Soc., 2010, 132, 28–29 CrossRef CAS.
- N. Shangguan, S. Katukojvala, R. Greenberg and L. J. Williams, J. Am. Chem. Soc., 2003, 125, 7754–7755 CrossRef CAS.
- A. Lindgren and T. Nilsson, Acta Chem. Scand., 1973, 27, 888–890 CrossRef.
- (a) G. A. Kraus and M. J. Taschner, J. Org. Chem., 1980, 45, 1175–1176 CrossRef CAS; (b) G. A. Kraus and B. Roth, J. Org. Chem., 1980, 45, 4825–4830 CrossRef CAS.
- B. S. Bal, W. E. Childers Jr. and H. W. Pinnick, Tetrahedron, 1981, 37, 2091–2096 CrossRef CAS.
- M. A. Mohamed, K. Yamada and K. Tomioka, Tetrahedron Lett., 2009, 50, 3436–3438 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental procedures and spectroscopic data. See DOI: 10.1039/c2ra20773g/ |
| ‡ Aldehyde (0.10 mmol, 1 equiv.), amine (0.15 mmol, 1.5 equiv.) and 2,3-dimethylbut-2-ene (0.50 mmol, 5 equiv.) were added into toluene (0.5 mL) and allowed to stir for 5 min. NaClO2 (0.3125 mmol, 3.125 equiv.) and NaH2PO4 (0.35 mmol, 3.5 equiv.) were then added to the reaction mixture. The reaction mixture was allowed to stir at 40 °C for 15–26 h and monitored by TLC. After the reaction was completed, the reaction mixture was diluted with anhydrous diethyl ether (5 mL), followed by adding saturated K2CO3 (5 mL). The aqueous layer was extracted with anhydrous diethyl eher (3 × 10 mL). The combined organic layers were dried over anhydrous sodium sulphate and the solvent was removed in vacuo. The crude product was directly loaded into a short silica gel column, followed by gradient elution with hexane/ethyl acetate mixtures (20/1 to 2/1 ratio). After removing the solvent, amide product was obtained. |
| § Reactions were performed using aldehyde (0.10 mmol) and amine (0.15 mmol) in 0.5 ml of solvent. |
| ¶ Isolated yield. |
|| Solvent used was Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA (4 EA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) due to the poor solubility of 4-hydroxybenzaldehyde. 1) due to the poor solubility of 4-hydroxybenzaldehyde. |
| ** 5 equiv. of NaClO2 and 4 equiv. of NaH2PO4 were used. |
| This journal is © The Royal Society of Chemistry 2012 |