Continuous photo-hydrogen production in anaerobic fluidized bed photo-reactor with activated carbon fiber as carrier†
Hong-Yu
Ren
,
Bing-Feng
Liu
*,
Jie
Ding
,
Guo-Jun
Xie
,
Lei
Zhao
,
De-Feng
Xing
,
Wan-Qian
Guo
and
Nan-Qi
Ren
*
State Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, P.O. Box 2614, 202 Haihe Road, Harbin 150090, China. Fax: +86 451 86282008; Tel: +86 451 86282110E-mail: rnq@hit.edu.cn; jianfeibio@yahoo.com.cn
First published on 30th April 2012
Abstract
A novel designed anaerobic fluidized bed photo-reactor (AFBPR) was applied in photo-hydrogen production using Rhodopseudomonas faecalis RLD-53 by continuous culture. The activated carbon fiber (ACF) was used as a support material to immobilize photo-fermentative bacteria for improving hydrogen production. This photo-reactor gave a fairly good and stable long-term performance.
The worldwide energy crisis and environmental concerns encourages people to seek alternative and sustainable energy sources.1,2 Hydrogen is carbon free and has an energy content per unit weight of 142 kJ g−1, which is much higher than those of fossil fuels.3 It is universally accepted as a renewable energy source and one of the potential solutions for current energy and environmental problems.4,5 However, currently hydrogen gas is mainly produced by thermo-chemical processes, such as coal gasification, hydrocarbon reforming, and partial oxidation of hydrocarbons.6 These methods must use fossil fuels as the raw material and are not friendly to environment. Among various technologies of hydrogen production, bio-hydrogen production seems to be a promising way to replace fossil fuels, owing to its potential to be an inexhaustible, low-cost and renewable energy source.6,7
Recently, bio-hydrogen production by photo-fermentative bacteria has been considered as a promising method to produce hydrogen.8 This is because photo-fermentation has a high energy conversion efficiency and can utilize a wide variety of metabolites, such as acetate and butyrate, which are the major by-products of dark fermentation.9,10 Hydrogen production by photo-fermentative bacteria has been studied with various types of photo-reactors.11–13 However, the major drawbacks of large-scale photo-hydrogen production are the low cell concentration and poor hydrogen production performance of the photo-reactors.8,12,14 Thus, there is an urgent need to develop a novel photo-reactor to overcome these problems.
An anaerobic fluidized bed reactor (AFBR) with an attached biofilm has been successfully applied in the fields of dark fermentation, and researchers have demonstrated the high efficiency of AFBRs for dark fermentation.15–19 Certain characteristics of the AFBR are beneficial to the hydrogen production process. These characteristics include the use of particles in a fluidized state to increase the effective area for bacterial immobilization; the ability to handle high organic loading rates; the high level of hydrogen producer that accumulates on the carrier; the better mixture conditions to promote the mass transfer efficiency; the low hydraulic retention time required.16,17,19 Though AFBRs have favorable characteristics for dark fermentation, they are rarely used in the fields of photo-fermentation. In addition, it is critical that AFBRs possess satisfactory mixing characteristics, which can avoid the formation of stagnant and dead zones and increase the mass transfer between substrate and bacterial cells, and favor hydrogen detachment.16,17
In the present work, the AFBPR had an effluent tube in its side wall. The effluent tube was declining to prevent carrying over of suspended particles into the effluent, and this design was different from the AFBRs used in dark fermentation.15–19 In addition, with the use of such a tube, the biogas and soluble products can be collected separately. As a result, there was no need to employ a gas–liquid separator commonly used in most AFBRs, which made the setup simpler.
The shading effect could cause light limitation and greatly decrease the hydrogen production performance of photo-reactor.20 Thus, the concentration of ACF was optimized before it was applied in the AFBPR (data not published). Furthermore, fluidization of immobilized particles can dilute the light and distribute it over the whole photo-reactor, so that bacterial cells were exposed to the light only for a short time, and this was good for increasing the light conversion efficiency and preventing light limitation.6
Therefore, the present study focused on continuous hydrogen production using a novel AFBPR (Fig. 1). The effects of the main operational parameters, including influent carbon source concentration, hydraulic retention time (HRT), and influent pH, were investigated to identify the favorable parameter variables for photo-hydrogen production. In addition, the performance of the AFBPR system was also evaluated in the present work.
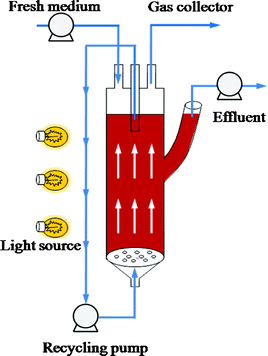 | ||
| Fig. 1 Schematic diagram of the anaerobic fluidized bed photo-reactor. | ||
Cell immobilization can enhance the activity of microorganisms and increase the biomass retention in the photo-reactor.21,22 In addition, research has showed that bacterial cells on carriers can decrease the mass transfer limitation between the substrate and bulk liquid, which can favor the improvement of photo-hydrogen production.22 Several carriers, such as granule activated carbon, silica gel, expanded clay, and porous glass,23,24 have been used to immobilize photo-fermentative bacteria. However, most carriers have a high density and cannot fluidize in the photo-reactor.24 This means that the cells cannot receive the light energy and so they just consumed the substrate, which decreased the hydrogen production performance.
ACF had a high specific surface area, excellent biocompatibility and good adsorption capacity.22,25 Furthermore, ACF could fluidize in the reactor and thus the hydrogen producer can absorb enough light energy, which is good for improving the light energy utilization and hydrogen production. In our previous work, ACF carriers can immobilize many bacterial cells on the surface and served excellently in photo-fermentative hydrogen production by batch culture.22 For further application, ACF carriers were used as support materials in the continuous AFBPR to produce hydrogen. A scanning electron microscope was applied to observe the surface of the ACF particles before and after the bacterial immobilization (Fig. 2). The SEM micrographs showed that particles of ACF were appropriate carriers for cell immobilization in the continuous culture. The surface of ACF was well covered by the cells, and the cell concentration was 0.85–1.37 g L−1 (Fig. 5b). In a previous study, the cell concentration was about 0.7–0.8 g L−1 in the suspended culture.26 This demonstrated that ACF immobilization was beneficial to promoting the cell concentration in the reactor.
 | ||
| Fig. 2 SEM images of the surface of ACF: (a) before immobilization and (b) after immobilization (magnification: 5000×). | ||
The carbon source concentration was a critical limiting factor that influenced the fermentation kinetics of the photo-hydrogen production.27 In the continuous operation, acetate was used as the sole carbon source because it was known as one of the major liquid metabolites of dark fermentation.28 To examine the effect of the carbon source concentration on the performance of photo-hydrogen production, different acetate concentrations were fed into the continuous photo-reactors (Fig. 3). The influent acetate concentrations used in this test were set at 25, 50 and 75 mmol L−1, respectively. It can be clearly observed that both the hydrogen production rate and yield reached a steady state within six days.
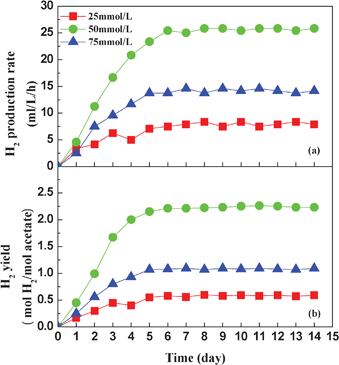 | ||
| Fig. 3 Effect of influent acetate concentration on hydrogen production rate (a) and hydrogen yield (b). | ||
The hydrogen production rate and yield increased with increasing acetate concentration and achieved their maximum values at an acetate concentration of 50 mmol L−1. Accordingly, the highest hydrogen production rate and yield were 25.8 mL L−1 h−1 and 2.26 mol H2/mol acetate, respectively. According to the principle of mass transfer,29 when the photo-fermentative bacteria had formed stable biofilm, the whole photo-reactor can be divided into two parts. One part was the bulk flow zone, and the other part was the biofilm zone. The substrate transport was determined by the carbon source concentration gradient between the two zones, and the substrate must transport from the bulk flow zone to the biofilm zone. It can be inferred that both the high acetate concentration and the fluidization of immobilized ACF particles could lead to a large concentration gradient between the two zones. This gradient could improve the mass transfer and result in a high acetate concentration in the biofilm zone. When the influent acetate concentration was 25 mmol L−1, the carbon source was consumed almost completely on the fourth day, so both the hydrogen production rate and the yield declined slightly. This indicated that the substrate was insufficient at the end of batch operation. The hydrogen production performance recovered quickly after feeding, which implied that the metabolic capacity of bacterial cells in the photo-reactor was sufficient to utilize the acetate and produce hydrogen when the influent substrate concentration was low. Under such conditions, the hydrogen production was governed by the mass transfer, and thus the amount of produced hydrogen increased with the increase of acetate concentration. Nevertheless, while the influent acetate concentration further increased to 75 mmol L−1, the hydrogen production rate and yield decreased to 14.2 mL L−1 h−1 and 1.08 mol H2/mol acetate, respectively. This outcome can be attributed to the inhibitory effect on the cells at high acetate concentrations. With the acetate concentration in the bulk flow zone increasing, the acetate transported to the biofilm zone could be accelerated. If the acetate consumption rate was lower than the acetate transport rate, the redundant acetate can accumulate inside the biofilm zone. Excessive amounts of acetate caused high penetration stress on the cells and ultimately inhibited the decomposition of acetate to hydrogen by the hydrogen producer. These results demonstrated that both a too low and too high influent acetate concentration resulted in the poor hydrogen production performance of the photo-reactor. Similar findings were also reported by other researchers.14
HRT was another key factor and could strongly affect the photo-fermentative hydrogen production in the photo-reactor.6 In this test, three different HRTs (36, 48 and 60 h) were used to investigate their effect on the continuous hydrogen production by the AFBPR (Fig. 4). Feeding started after four days of batch culture. The experimental results showed that at the HRT of 36 and 48 h, the photo-reactors reached a stable state on the fifth day in terms of hydrogen production rate and yield, while at the HRT of 60 h the photo-reactor only obtained a stable state on the seventh day. The hydrogen production rate increased with decreasing HRT and attained the maximum value of 28.3 mL L−1 h−1 at the HRT of 36 h. This phenomenon could be explained because the mass transfer rate of the substrate dominated the biochemical reaction rate, so a short HRT usually leads to a high organic load rate, causing an improvement in the mass transport from the bulk flow zone to the biofilm zone on the immobilized ACF particles. Therefore, the hydrogen production rate of the photo-reactor was improved. However, the hydrogen yield of HRT 36 h was lower than that of HRT 48 h. This drop was due to the possibility of undesirable loss of activated cells in the photo-reactor resulting from the short HRT, and thus more substrate was available and was used for bacterial growth instead of hydrogen production. The acetate concentration in the effluent when the HRT was 36 h was higher than that of other HRTs (Fig. 5a), and this resulted in the low utilization rate of acetate during the hydrogen production process. At the long HRT of 60 h, the cell concentration was 1.37 g L−1 (Fig. 5b), which was higher than the cell concentration of HRT 36 h (0.85 g L−1) and HRT 48 h (1.11 g L−1). These values were much higher than those achieved from the suspension system.26 However, at the long HRT of 60 h, not only the hydrogen production rate but also the hydrogen yield were in low levels. This implied the slow conversion rate of VFAs to H2 and CO2 by the strain RLD-53. When the HRT was long, the update of the culture medium was slow and more hydrogen producer was maintained in the photo-reactor. However, redundant bacterial cells were detrimental for the hydrogen production, because most of them were aged cells in the decline phase and so they consumed the substrate for maintenance purposes rather than hydrogen production. Thus, a high cell concentration and low effluent acetate concentration occurred at HRT 60 h, and so the hydrogen production rate and yield were only 14.6 mL L−1 h−1 and 1.36 mol H2/mol acetate, respectively. These findings correlated with the work of Androga et al.36
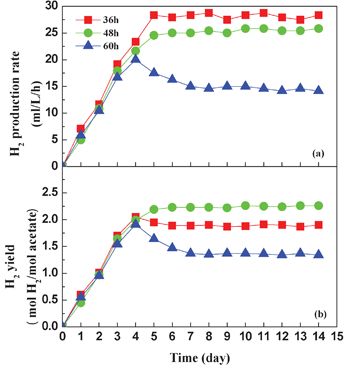 | ||
| Fig. 4 Effect of HRT on hydrogen production rate (a) and hydrogen yield (b). | ||
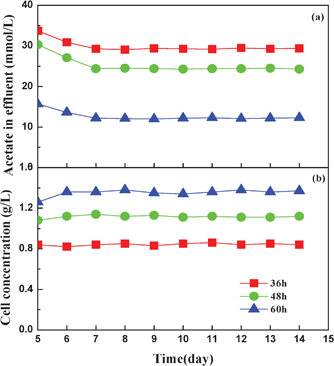 | ||
| Fig. 5 The change of effluent acetate concentration (a) and cell concentration (b) under different HRTs. | ||
The above results demonstrated that the short or long HRTs for the substrate in the photo-reactor were insufficient for efficient hydrogen bioconversion, even though the hydrogen production rate or cell concentration was higher. The highest hydrogen yield of 2.26 mol H2/mol acetate was obtained at HRT of 48 h. This value was higher than most reported values (Table 1). Therefore, an optimal HRT of 48 h was adopted for photo-hydrogen production by the AFBPR in the following experiments.
| Microorganism | Substrate | Concentration | Light source | H2 yieldb | H2 production rate (mL L−1 h−1) | Light intensity | References |
|---|---|---|---|---|---|---|---|
| a NA, not available. b H2 yield, mol H2/mol substrate. c TL, Tungsten lamp. d OF, Optical fiber. e HL, Halogen lamp. f Calculated value based on the reported data. | |||||||
| R. sphaeroides RV | Lactate | 100 mmol L−1 | TLc | 4.8 | 66.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux 000 lux |
30 |
| R. rubrum | Lactate | 20 mmol L−1 | TL | 1.13 | 49.7 | 130 mW cm−2 | 31 |
| R. sphaeroides KD131 | Lactate | 20 mmol L−1 | TL | 2.3 | 12.88 | 110 W m−2 | 32 |
| R. sphaeroides O.U.001 | Malate | 7.5 mmol L−1 | TL | NAa | 20 | 200 W m−2 | 33 |
| R. sphaeroides O.U.001 | Malate | 15 mmol L−1f | TL | 4.5 | 6.5 | 15 W m−2 | 34 |
| R. palustris CQK01 | Glucose | 120 mmol L−1 | LED | 0.2 | 38.9 | 5000 lux | 23 |
| R. palustris CQK01 | Glucose | 55.6 mmol L−1f | LED | 0.75 | NAa | 6.75 W m−2 | 29 |
| R. palustris CQK01 | Glucose | 60 mmol L−1 | OFd/HLe | NAa | 39.2f | 12 W m−2 | 14 |
| R. capsulatus DSM 1710 | Acetate | 40 mmol L−1 | Sunlight | 0.0007 | 7 | NAa | 13 |
| R. palustris WP 3–5 | Acetate | 1000 mg COD L−1 | OF/HL/TL | 3.37 | 40.6 | 95 W m−2 | 24 |
| R. palustris WP 3–5 | Acetate | 1000 mg COD L−1 | HL/TL | 2.4 | 20.9 | 95 W m−2 | 24 |
| R. palustris WP 3–5 | Acetate | 1000 mg COD L−1 | TL | 0.86 | 6.86 | 95 W m−2 | 35 |
| R. faecalis RLD-53 | Acetate | 50 mmol L−1 | TL | 2.26 | 25.8 | 150 W m−2 | This study |
Variation of the pH of the influent medium changed the ionic form of the active site and the enzyme activity.37 Consequently, biochemical reaction characteristics were commonly affected by the pH of the culture medium. In order to attain the performance of the photo-reactor at the optimal level, the pH of the influent medium should be adjusted to meet the metabolic needs of the photo-fermentative bacteria for hydrogen production. In the present study, experiments were conducted with different pHs of the influent solution (6.0, 7.0, and 8.0). Results showed that when the pH of the influent medium was 7.0, the hydrogen production rate and yield reached the maximum values (Fig. 6). This revealed that the acid or alkaline culture medium resulted in the inactivation of cellular enzymes and depressed the substrate decomposition and electron transfer, which caused the low hydrogen production performance. In a previous investigation, R. faecalis RLD-53 cannot grow and produce hydrogen at an initial pH of 6.0 in the suspension culture, which was because the low pH induced low levels of ATP in the cells and further inhibited the bacterial growth.38 However, a small amount of hydrogen was observed on the fourth day when the photo-reactor was run at influent pH of 6.0, and the photo-reactor could produce hydrogen at the rate of 3.3 mL L−1 h−1. The reason for this could be because the bacterial immobilization enhanced the acid-tolerance of the hydrogen producer and thus led to a slight improvement in the hydrogen production performance. Similar results were also found by investigating the hydrogen production performance of strain RLD-53 immobilized on agar gel granules by batch experiments.39 When the influent pH was 8.0, the hydrogen production rate was higher than that for pH 6.0, and attained a rate of 11.7 mL L−1 h−1 at the steady state. As a result, it can be concluded from the experiments that a neutral medium was the optimum conditions for photo-hydrogen production, and the optimal influent pH of 7.0 for AFBPR was comparable to the pH of 6.8 reported for R. sphaeroides O.U.00134 and pH of 7.0 for R. palustris CQK 01.23
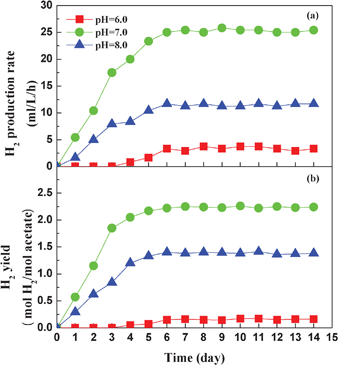 | ||
| Fig. 6 Effect of influent pH on the hydrogen production rate (a) and hydrogen yield (b). | ||
In the present work, the maximum hydrogen yield of 2.26 mol H2/mol acetate was comparable and satisfactory compared to most of the values reported in the literature, whereas it appeared to be lower than a few previously reported results (Table 1). The findings of previous work in the literature is shown in Table 1, and clearly points out that the light source was a critical factor for the bio-hydrogen production system, and the hydrogen production performance can be much enhanced by applying a more efficient illumination system.14,40 In this work, only a tungsten lamp was adopted as the external light source. Therefore, the hydrogen yield in this study was lower than that acquired using an OF/HL/TL system. Nevertheless, the hydrogen yield achieved in this work was still higher than most systems with a tungsten lamp as the light source, indicating that the AFBPR system developed in the present research was promising.
In conclusion, the novel anaerobic fluidized bed photo-reactor using ACF particles as the support material proved to be fairly stable and efficient for hydrogen production. This study demonstrated that HRT, influent acetate, and influent pH all played crucial roles in affecting the hydrogen production performance of the AFBPR. It can be concluded from experimental results that the optimum operational conditions for hydrogen production in the AFBPR were: HRT 48 h, influent acetate concentration 50 mmol L−1, and influent pH 7.0. Under such conditions the maximum hydrogen yield and hydrogen production rate were 2.26 mol H2/mol acetate and 25.8 ml L−1 h−1, respectively. This performance of the AFBPR was competitive to the results of other studies, indicating the feasibility and potential of applying the developed photo-reactor system to large-scale photo-hydrogen production.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant no. 51178140 and 51106040), “the Fundamental Research Funds for the Central Universities” (grant no. HIT. NSRIF. 201186 and 2009115), China Postdoctoral Science Foundation (grant no. 20110491054), Heilongjiang Postdoctorial Financial Assistance (no. LBH-Z11120), project 51121062 (National Creative Research Groups) and the National High Technology Research and Development Program of China (863 Program) (grant no. 2011AA060905).References
- D. Pant, A. Singh, G. Van Bogaert, S. Irving Olsen, P. Singh Nigam, L. Diels and K. Vanbroekhoven, RSC Adv., 2012, 2, 1248–1263 RSC.
- N. Ren, B. Wang and J. C. Huang, Biotechnol. Bioeng., 1997, 54, 428–433 CrossRef CAS.
- D. Das, Int. J. Hydrogen Energy, 2009, 34, 7349–7357 CrossRef CAS.
- M. A. Basile, C. Carfagna, P. Cerruti, G. Gomez d'Ayala, A. Fontana, A. Gambacorta, M. Malinconico and L. Dipasquale, RSC Adv., 2012, 2, 3611–3614 RSC.
- A. S. Joshi, I. Dincer and B. V. Reddy, Int. J. Hydrogen Energy, 2011, 36, 11246–11257 CrossRef CAS.
- K.-Y. Show, D.-J. Lee and J.-S. Chang, Bioresour. Technol., 2011, 102, 8524–8533 CrossRef CAS.
- N. Ren, W. Guo, B. Liu, G. Cao and J. Ding, Curr. Opin. Biotechnol., 2011, 22, 365–370 CrossRef CAS.
- C. N. Dasgupta, J. Jose Gilbert, P. Lindblad, T. Heidorn, S. A. Borgvang, K. Skjanes and D. Das, Int. J. Hydrogen Energy, 2010, 35, 10218–10238 CrossRef CAS.
- H.-Y. Ren, B.-F. Liu, J. Ding, J. Nan, G.-J. Xie, L. Zhao, M.-G. Chen and N.-Q. Ren, Int. J. Hydrogen Energy, 2012, 37, 8277–8281 CrossRef CAS.
- J. Cai, G. Wang and G. Pan, Int. J. Hydrogen Energy, 2012, 37, 4057–4067 CrossRef CAS.
- C.-M. Lee, G.-J. Hung and C.-F. Yang, Bioresour. Technol., 2011, 102, 8350–8356 CrossRef CAS.
- J. J. Gilbert, S. Ray and D. Das, Int. J. Hydrogen Energy, 2011, 36, 3434–3441 CrossRef CAS.
- E. Boran, E. Ozgur, J. Gebicki, J. van der Burg, M. Yucel, U. Giindiiz, M. M. I. Eroglu and T. B. V. Netherlands, Chem. Eng. Trans., 2009, 18, 357–362 Search PubMed.
- C.-L. Guo, X. Zhu, Q. Liao, Y.-Z. Wang, R. Chen and D.-J. Lee, Bioresour. Technol., 2011, 102, 8507–8513 CrossRef CAS.
- S.-C. Kuo, Y.-C. Chao, Y.-M. Tien, I. C. Chen and S.-S. Cheng, Int. J. Hydrogen Energy, 2011, 36, 8800–8808 CrossRef CAS.
- C. M. dos Reis and E. L. Silva, Chem. Eng. J., 2011, 172, 28–36 CAS.
- G. M. Shida, A. R. Barros, C. M. d. Reis, E. L. C. d. Amorim, M. H. Rissato Zamariolli Damianovic and E. L. Silva, Int. J. Hydrogen Energy, 2009, 34, 3679–3688 CrossRef CAS.
- E. L. Cavalcante de Amorim, A. R. Barros, M. H. Rissato Zamariolli Damianovic and E. L. Silva, Int. J. Hydrogen Energy, 2009, 34, 783–790 CrossRef CAS.
- Z.-P. Zhang, J.-H. Tay, K.-Y. Show, R. Yan, D. Tee Liang, D.-J. Lee and W.-J. Jiang, Int. J. Hydrogen Energy, 2007, 32, 185–191 CrossRef CAS.
- G.-J. Xie, B.-F. Liu, J. Ding, H.-Y. Ren, D.-F. Xing and N.-Q. Ren, Int. J. Hydrogen Energy, 2011, 36, 13991–13996 CrossRef CAS.
- L. Zhao, G.-l. Cao, A.-j. Wang, W.-q. Guo, B.-f. Liu, H.-y. Ren, N.-q. Ren and F. Ma, Int. J. Hydrogen Energy, 2012, 37, 162–166 CrossRef CAS.
- G.-J. Xie, B.-F. Liu, D.-F. Xing, J. Nan, J. Ding, H.-Y. Ren, W.-Q. Guo and N.-Q. Ren, RSC Adv., 2012, 2, 2225–2228 RSC.
- X. Tian, Q. Liao, X. Zhu, Y. Wang, P. Zhang, J. Li and H. Wang, Bioresour. Technol., 2010, 101, 977–983 CrossRef CAS.
- C.-Y. Chen and J.-S. Chang, Process Biochem., 2006, 41, 2041–2049 CrossRef CAS.
- G.-J. Xie, B.-F. Liu, W.-Q. Guo, J. Ding, D.-F. Xing, J. Nan, H.-Y. Ren and N.-Q. Ren, Int. J. Hydrogen Energy DOI:10.1016/j.ijhydene.2012.02.107.
- N.-Q. Ren, B.-F. Liu, J. Ding, W.-Q. Guo, G.-L. Cao and G.-J. Xie, Int. J. Hydrogen Energy, 2008, 33, 5981–5985 CrossRef CAS.
- H. H. P. Fang, H. Liu and T. Zhang, Int. J. Hydrogen Energy, 2005, 30, 785–793 CrossRef CAS.
- K. Nath, A. Kumar and D. Das, Appl. Microbiol. Biotechnol., 2005, 68, 533–541 CrossRef CAS.
- C. Zhang, X. Zhu, Q. Liao, Y. Wang, J. Li, Y. Ding and H. Wang, Int. J. Hydrogen Energy, 2010, 35, 5284–5292 CrossRef CAS.
- E. Fascetti and O. Todini, Appl. Microbiol. Biotechnol., 1995, 44, 300–305 CrossRef CAS.
- P. von Feiten, H. Zürrer and R. Bachofen, Appl. Microbiol. Biotechnol., 1985, 23, 15–20 CrossRef.
- D.-H. Kim, H. Son and M.-S. Kim, Int. J. Hydrogen Energy DOI:10.1016/j.ijhydene.2012.02.039.
- İ. Eroğlu, K. Aslan, U. Gündüz, M. Yücel and L. Türker, in BioHydrogen, ed. O. R. Zaborsky, Plenum Press, NY, 1998, pp. 143–149 Search PubMed.
- N. Basak and D. Das, Biomass Bioenergy, 2009, 33, 911–919 CrossRef CAS.
- K.-L. Yeh, C.-Y. Chen, Y.-C. Lo and J.-S. Chang, Proceedings WHEC2010, 2010, 78, 61–65 Search PubMed.
- D. D. Androga, E. Özgür, I. Eroglu, U. Gündüz and M. Yücel, Int. J. Hydrogen Energy, 2011, 36, 15583–15594 CrossRef CAS.
- C.-Y. Chen, C.-H. Liu, Y.-C. Lo and J.-S. Chang, Bioresour. Technol., 2011, 102, 8484–8492 CrossRef CAS.
- N.-Q. Ren, B.-F. Liu, J. Ding and G.-J. Xie, Bioresour. Technol., 2009, 100, 484–487 CrossRef CAS.
- B.-F. Liu, G.-J. Xie, W.-Q. Guo, J. Ding and N.-Q. Ren, Nat. Resour., 2011, 2, 1–7 Search PubMed.
- C.-Y. Chen, C.-M. Lee and J.-S. Chang, Int. J. Hydrogen Energy, 2006, 31, 2345–2355 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20420g/ |
| This journal is © The Royal Society of Chemistry 2012 |
